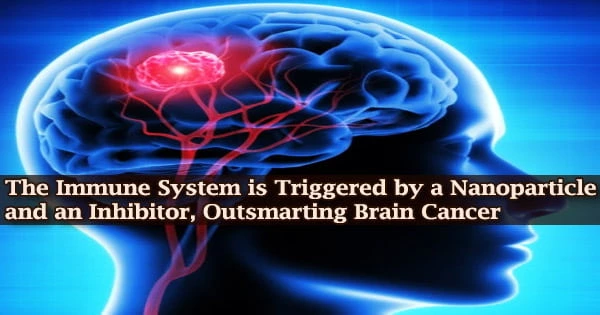
When researchers at the University of Michigan’s Rogel Cancer Center discovered a tiny chemical that blocked a key mechanism in brain tumors, they were optimistic. But there was a problem: getting the inhibitor into the brain and via the bloodstream to reach the tumor.
The researchers created a nanoparticle to encapsulate the inhibitor in coordination with multiple labs, and the results were even better than expected.
The nanoparticles not only delivered the inhibitor to the tumor in mouse models, where the drug successfully turned on the immune system to eliminate the cancer, but the process also triggered immune memory, indicating that this potential new approach could not only treat brain tumors but also prevent or delay recurrences.
“No one could get this molecule into the brain. It’s really a huge milestone. Outcomes for patients with glioma have not improved for the last 30 years,” said Maria G. Castro, Ph.D., R.C. Schneider Collegiate Professor of Neurosurgery at Michigan Medicine. Castro is the senior author of the study, published in ACS Nano.
“Despite survival gains in many cancer types, glioma remains stubbornly challenging, with only 5% of patients living five years after their diagnosis,” said study author Pedro R. Lowenstein, M.D., Ph.D., Richard C. Schneider Collegiate Professor of Neurosurgery at Michigan Medicine.
No one could get this molecule into the brain. It’s really a huge milestone. Outcomes for patients with glioma have not improved for the last 30 years.
Maria G. Castro
Traditional medicines are frequently ineffectual against gliomas, and the environment inside the tumor suppresses the immune system, making new immune-based therapies useless. When you include in the difficulty of getting through the blood-brain barrier, it becomes much more difficult to treat these tumors effectively.
The Castro-Lowenstein lab spotted a chance. AMD3100 is a small molecule inhibitor that works by blocking the action of CXCR12, a cytokine generated by glioma cells that forms a protective shield around the immune system, preventing it from attacking the invading tumor.
AMD3100 blocked CXCR12 from associating with immune-suppressive myeloid cells in mice models of glioma, according to researchers. The immune system is preserved by disarming these cells, allowing it to assault cancerous cells.
However, AMD3100 was having difficulty reaching the tumor. The medicine did not travel well through the bloodstream and did not cross the blood-brain barrier, which is a major barrier to drug delivery to the brain.
The Castro-Lowenstein lab worked with Joerg Lahann, Ph.D., Wolfgang Pauli Collegiate Professor of Chemical Engineering at the University of Michigan College of Engineering, to develop protein-based nanoparticles that would encapsulate the inhibitor and allow it to pass through the bloodstream more easily.
Castro also met with Anuska V. Andjelkovic, M.D., Ph.D., a Michigan Medicine professor of pathology and research professor of neurosurgery whose research focuses on the blood-brain barrier. Glioma tumors produce aberrant blood arteries, which obstruct normal blood flow, according to the researchers.
The researchers used AMD3100-loaded nanoparticles to inject into glioma-infected mice. On the surface of the nanoparticles was a peptide that binds to a protein found predominantly on brain tumor cells.
The nanoparticles released AMD3100 as they moved through the bloodstream toward the tumor, restoring the blood vessel integrity. The nanoparticles could then reach their target and release the medication, preventing immune-suppressive myeloid cells from entering the tumor mass. The immune cells were able to attack the tumor and slow its progression as a result of this.
“If you don’t have blood flow, nothing will get to your target. That’s why tumors are so smart. But AMD3100 restores the conduits, which is what allows the nanoparticles to reach the tumor,” Castro said.
More research in animals and patient cell lines revealed that combining the AMD3100 nanoparticle with radiation therapy improved the effect over either the nanoparticle or radiation alone.
The researchers then implanted the tumor into the mice who had been removed, imitating a recurrence. Sixty percent of mice remained cancer-free without any extra treatment.
This shows that AMD3100 induced immunological memory, allowing the immune system to recognize and eliminate the reintroduced cells, much like a vaccine. While it prevented recurrence in mice, Castro believes it will at the very least postpone recurrence in humans.
“Every glioma recurs. It’s very important for glioma therapy to have this immunological memory,” Castro said.
Initial testing revealed that the medication had little to no effect on the mice’s liver, kidney, or heart function, as well as normal blood counts. The nanoparticle’s basis is similar to that of nanoparticles that have been examined in people and found to be safe. Before moving forward with a clinical trial, more safety testing is required.