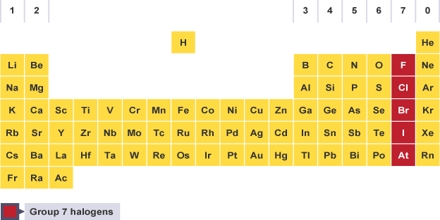
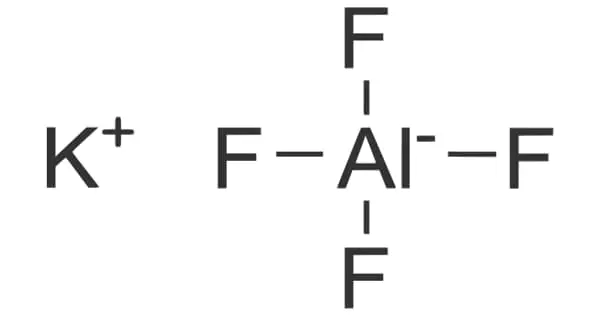
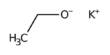
General objective of this lecture is to present on the Halogens. The halogens are a group of non- metals in the periodic table. They all have seven electrons in their outer shell this makes them all really reactive; they only have to gain one more electron to fill their outer shell. Unlike Group One the elements get less reactive as you go down the group. Here briefly explain on the Halogens family Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), Astatine (At) and uses.
The Halogens