According to one component of migraine pain hypothesis, migraine pain is caused by waves of activity by groupings of excitable brain cells. These cause neurotransmitters like serotonin to constrict blood arteries. Serotonin is a neurotransmitter that is required for nerve cell transmission.
Schwann cells, which are widespread in the peripheral nervous system and form a protective sheath around nerve fibers, have been revealed to have an important role in migraine pain by an international team of researchers. Their research, which was carried out in mice and human Schwann cells and published in Nature Communications, shows how pain is communicated from within Schwann cells and identifies numerous strategies to inhibit this communication, suggesting possible targets for new migraine treatments.
Migraines impact more than 15% of adults, with women being twice as likely as men to suffer from these severe headaches. Calcitonin gene-related peptide (CGRP), a tiny protein in the neurological system, is recognized to have an essential role in migraine pain; in fact, a new generation of migraine medications uses monoclonal antibodies to target CGRP or its receptor.
While CGRP has been implicated in migraine pain, how it causes pain has been an area of controversy in the scientific community. The efficacy of CGRP monoclonal antibodies for migraine and the inability of antibodies to pass the blood-brain barrier suggest that CGRP produces pain in the periphery rather than within the brain.
Professor Nigel Bunnett
“While CGRP has been implicated in migraine pain, how it causes pain has been an area of controversy in the scientific community,” said Nigel Bunnett, Ph.D., professor and chair of the Department of Molecular Pathobiology at NYU College of Dentistry. Bunnett led the study with Pierangelo Geppetti, MD, professor of clinical pharmacology at the University of Florence and director of the Headache Center of Careggi University Hospital.
“The efficacy of CGRP monoclonal antibodies for migraine and the inability of antibodies to pass the blood brain barrier suggest that CGRP produces pain in the periphery rather than within the brain,” Bunnett, an NYU Pain Research Center researcher, stated.
Bunnett and his colleagues focused on Schwann cells, which are present outside the brain in the peripheral nervous system, to investigate the biological mechanism of CGRP-evoked pain. The researchers looked at mice that had the CGRP receptor, known as CLR/RAMP1, deactivated in Schwann cells. They altered the CGRP receptor by removing RAMP1, one of the receptor’s two key components, from Schwann cells in the mouse’s face.
CGRP administration made the facial region of normal mice extremely sensitive, acting as a surrogate for migraine pain. CGRP, on the other hand, did not elicit pain in animals lacking the CGRP receptor in Schwann cells. In a subsequent experiment, the researchers used capsaicin, a substance found in hot chili peppers. Capsaicin activates an ion channel called TRPV1, which produces pain by releasing CGRP. Again, capsaicin did not elicit migraine-like pain in animals lacking the CGRP receptor in Schwann cells, indicating that the CGRP receptor in Schwann cells is important in migraine pain.
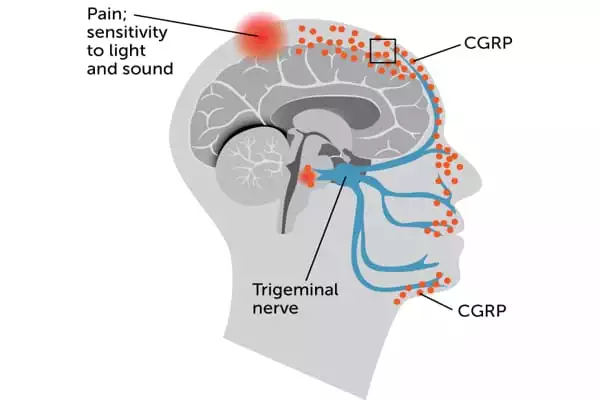
The researchers then uncovered what happens within human Schwann cells to signal pain. When CGRP attaches to its receptor on a Schwann cell, the receptor travels into a compartment within the cell known as an endosome. The CGRP receptor continues to signal for extended periods of time within endosomes. This transmission produces nitric oxide, a pain mediator, which is then released from the Schwann cell and interacts with an ion channel on an adjacent neuron called TRPA1. TRPA1 stimulates the neuron and sends pain signals.
This new understanding of how pain begins within Schwann cells provided the researchers with two treatment options for migraine pain: preventing the CGRP receptor from accessing endosomes in the first place, or employing nanoparticles to deliver medications targeting the CGRP receptor in endosomes.
The researchers blocked clathrin and dynamin, two proteins involved in the transport of chemicals into cells, to keep CGRP receptors out of endosomes. Clathrin and dynamin inhibition reduced pain signaling, suggesting a possible target for novel migraine therapies.
The researchers were also successful in blocking CGRP-evoked pain by encapsulating a small molecule medication that binds to and disables the CGRP receptor in nanoparticles. While most medications only reach the cell’s surface, nanoparticles can be created to assist medicine in reaching the endosome inside a cell and releasing the drug once it arrives. The nanoparticles transported the medication into endosomes and blocked the CGRP receptor, thereby inhibiting migraine pain in Schwann cells.
“While the significance of CGRP in migraine pain is widely established, this is the first study to specifically link Schwann cells to migraine pain. Based on our improved understanding of how pain is signaled from within endosomes, it offers prospective new techniques to treating migraine “Bunnett stated.
The researchers are collaborating with many partners, including the National Institutes of Health’s National Center for Advancing Translational Sciences, to continue exploring the use and safety of nanoparticle drug delivery before these treatments may be tried in humans.