The process of drying could have played a role in the development of life on Earth. Water is a necessary component for the chemical reactions that are thought to have led to the development of life, and drying could have helped to concentrate these chemicals and facilitate their interaction. The process of drying can also help to preserve biological materials, which could have allowed early forms of life to survive and evolve. In addition, drying can lead to the formation of microenvironments that could have supported the development of more complex forms of life. Overall, the process of drying could have been an important factor in the emergence and evolution of life on Earth.
New research could help explain crucial early steps on the path of life, from a pool of simple amino acids to bacteria, redwood trees, and humans. Charles Darwin proposed 150 years ago that life most likely began in a warm little pond. There, Darwin hypothesized, chemical reactions and the occasional lightning strike might have resulted in chains of amino acids that grew more complex over time until the beginnings of life emerged.
Since then, scientists have been investigating this type of pre-life or “prebiotic” chemistry, attempting to deduce the chemical pathways that could have led from a pool of simple amino acids to bacteria, redwood trees, and humans. Following a series of experiments, Hayley Boigenzahn, a Ph.D. student in chemical engineering at the University of Wisconsin-Madison, and John Yin, a professor of chemical and biological engineering and a founding faculty member of the Wisconsin Institute for Discovery, can explain how one of the potentially critical early steps on the path of life could have occurred. They published their findings in the journal Origins of Life and Evolution of Biospheres in December 2022.
DNA can store information at a density thousands of times that of a computer chip. If we can create systems that can do this without necessarily being living cells, you can imagine all kinds of new molecular functions and processes.
John Yin
The Miller-Urey experiment, conducted in 1952, simulated the conditions thought to exist on the prebiotic Earth, including specific ratios of water, methane, hydrogen, and other elements. When the reaction was zapped with electricity to simulate lightning, the researchers discovered that amino acids were produced, implying that these molecules were common on prebiotic Earth.
“We know that amino acids are the building blocks of proteins, and proteins are necessary for life,” Yin explains. “The question in prebiotic chemistry has long been how to get these things to form bonds and strings in a way that could eventually lead to a living cell. The question is difficult because the chemistry involved is one that fails in the presence of water.”
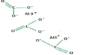
In her experiment, Boigenzahn investigated whether it’s possible these amino acids could have come together during periods of environmental change – for instance, as a pool of water evaporated. In the presence of a chemical activator, these amino acids could bond together into peptides, or short chains of amino acids.
Boigenzahn created solutions of the amino acid glycine and trimetaphosphate, an activator naturally produced during volcanic processes, to investigate how amino acids might form bonds during the drying process. Boigenzahn observed what happened to the amino acids over a 24-hour period while using a heater to evaporate the solution.
She discovered a two-stage process. When the pH of the solution was alkaline in the first stage, the glycine combined into two-molecule units called dimers, which also produced protons, making the pH of the solution neutral. As evaporation occurred in the second stage, the dimers began to bond together to form longer peptide chains known as oligoglycine.
It’s easy to imagine a scenario in which amino acids in a volcanically warmed hot spring containing an activator first combine into dimers. Then, as the water evaporates and its chemistry changes, the dimers bond and begin to form into longer chains of amino acids.
“What we’re demonstrating here is that it doesn’t have to be the same environment throughout all of the reactions,” says Boigenzahn. “They can occur in various environments, as long as the reactions that occur help to create an environment that is beneficial for the next steps.”
It’s possible that the peptide chains grew longer and longer after multiple wet-dry cycles. They may have eventually folded in on themselves, forming enzymes or proteins that catalyze chemical reactions. This could pave the way for more complex proteins and the start of metabolism.
Both Boigenzahn and Yin believe it will be a long time before scientists figure out how to get from Darwin’s warm little pond to the beginnings of life. However, for chemical engineers in particular, studying prebiotic chemistry could pay off handsomely.
“If you really understand this chemistry, which is different from traditional biology, you might be able to create chemical systems that can store information, adapt, and evolve,” Yin says. “DNA can store information at a density thousands of times that of a computer chip. If we can create systems that can do this without necessarily being living cells, you can imagine all kinds of new molecular functions and processes.”