Numerous characteristics of carbon-based materials make them desirable as catalysts for accelerating chemical reactions. They are inexpensive, light, and have a large surface area, which makes them an ideal scaffold for anchoring catalysts and keeping them stable and widely disseminated while giving molecules a lot of working surface area.
This makes carbons useful for energy storage and sensors. Carbons have been utilized in electrochemistry for the past ten years to catalyze processes that create compounds and fuel cells.
However, in work recently reported in Nature Communications, University of Delaware’s Dion Vlachos and researchers in the Catalysis Center for Energy Innovation (CCEI), with collaborators from Brookhaven National Laboratory, made some surprising findings as they were developing techniques to better understand the role oxygen plays in how carbon-based catalysts perform.
According to Vlachos, what they found turned some of what they knew about chemistry upside down.
Not all oxygens are the same
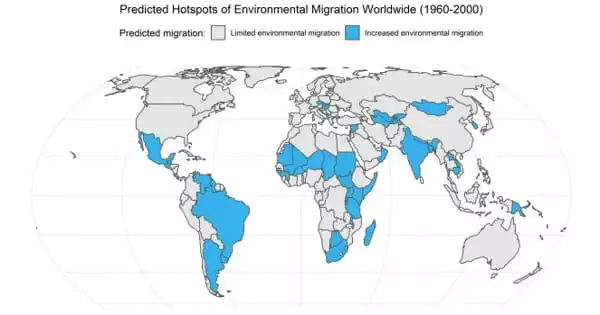
Despite their utility, carbons are not well understood. They are not uniform either. Oxygen can occasionally be found in carbon-based materials, and it can do so in a number of various ways, including as an alcohol, aldehyde, ketone, or acid. What the oxygen performs in these carbon compounds is one unanswered question.
So, using carbon molecules as a starting point, Vlachos and his team gradually added more and more oxygen. Then, they used spectroscopic methods to characterize the final substance and determine how much and what kind of oxygen was present.
The University of Delaware team accomplished an impressive feat by using advanced tools and methods to unravel a complicated catalytic system. We are delighted to have played a part in this significant achievement by conducting measurements using a special kind of spectroscopy at the Center for Functional Nanomaterials.
Anibal Boscoboinik
The scientists carried out the reactions employing the various oxygenated carbons for a library of roughly 10 to 15 items. This gave them the opportunity to use machine learning algorithms to correlate the reactivity of the carbon material with the quantity and kind of oxygens present.
The research of the team revealed a relationship between performance and the quantity and type of oxygen present, including which oxygens are more active. The researchers also discovered a paradoxical result: long-range impacts from aromatic rings far from a catalyst site can occasionally result in the carbon’s alcohol functional groups becoming more acidic than typical acidic carbon functional groups observed in organic chemistry small acids.
The impact was proved to be caused by the oxygenated carbons in aromatic rings, which are based on alcohol, after the researchers had initially been taken aback.
“Carbon has aromatic rings,” said Vlachos, the Unidel Dan Rich Chair in Energy and director of CCEI, an Energy Frontier Research Center supported by the U.S. Department of Energy. “And the more carbon rings that are added to a material, the greater the chance of creating a regional phenomenon where long-range effects from far away can have a controlling effect on the activity of the catalyst sites.”
This is not the case with typical catalysis chemistry, where the effect is very local. For example, bond A affects bond B and that’s it.
“The whole chemistry thinking is upside down. This was not expected,” he added.
In terms of applications, Vlachos said this means if researchers want to create a more acidic carbon catalyst, they will need to use more alcohol functional groups, in this case, hydroxyls.
The researchers to characterize what would happen to the oxygen in materials under conditions that were close to those found in the actual world and to validate the results of the mathematical modeling employed advanced approaches.
“The University of Delaware team accomplished an impressive feat by using advanced tools and methods to unravel a complicated catalytic system,” said Anibal Boscoboinik, a materials scientist with the Center for Functional Nanomaterials, a U.S. Department of Energy (DOE) Office of Science User Facility at DOE’s Brookhaven National Laboratory. “We are delighted to have played a part in this significant achievement by conducting measurements using a special kind of spectroscopy at the Center for Functional Nanomaterials.”
The research team can experiment with various methods for creating materials to discover which has the best impact using this new way for figuring out what each component of the chemistry is doing.
For example, are all oxygen molecules equally effective at speeding up catalytic reactions, or are some better than others? Vlachos is also interested in the possibility of dispersing metals for reactions using the oxygen supply. Finding environmentally friendly alternatives to the corrosive traditional methods for adding oxygen to a reaction to create materials could hasten the development of more sustainable technologies.
Jiahua Zhou and Piaoping Yang, chemical and biomolecular engineering doctoral students, served as co-lead authors on the paper. CCEI director Dion Vlachos and Weiqing Zheng, CCEI associate director, served as co-principal investigators on the project. UD co-authors include Stavros Caratzoulas, Pavel A. Kots and Caitlin M. Quinn.
Other collaborating co-authors include Matheus Dorneles de Mello and J. Anibal Boscoboinik from Brookhaven National Laboratory, and Ying Chen, Maximilian Cohen and Wendy J. Shaw, from Pacific Northwest National Laboratory.