A reflection of the growing understanding that immune processes play a critical role in causing the brain damage that results in confusion, memory loss, and other disabling symptoms, nearly two dozen experimental therapies that target the immune system are currently undergoing clinical trials for Alzheimer’s disease.
The indigenous immune cells in the brain called microglia, which can damage brain tissue if triggered at the incorrect time or in the incorrect manner, are the target of many of the immunity-focused Alzheimer’s medicines currently being developed.
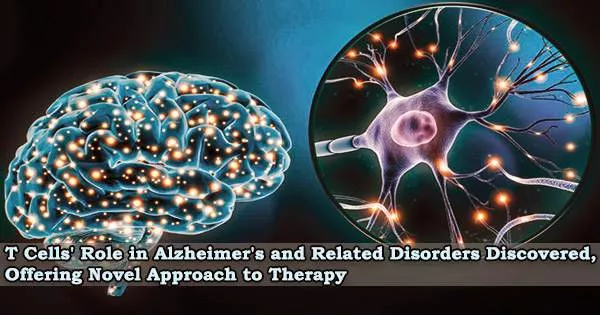
A new study from researchers at Washington University School of Medicine in St. Louis indicates that microglia partner with another type of immune cell T cells to cause neurodegeneration.
Researchers found that microglia draw potent cell-killing T cells into the brain of mice with brain damage similar to Alzheimer’s caused by the protein tau, and that most neurodegeneration could be prevented by inhibiting the T cells’ entry or activation.
The findings, published March 8 in the journal Nature, suggest that targeting T cells is an alternative route to preventing neurodegeneration and treating Alzheimer’s disease and related diseases involving tau, collectively known as tauopathies.
Scientists have put a lot of effort into finding therapies that prevent neurodegeneration by affecting tau or microglia. As a community, we haven’t looked at what we can do to T cells to prevent neurodegeneration. This highlights a new area to better understand and therapeutically explore.
David M. Holtzman
“This could really change the way we think about developing treatments for Alzheimer’s disease and related conditions,” said senior author David M. Holtzman, MD, the Barbara Burton and Reuben M. Morriss III Distinguished Professor of Neurology. “Before this study, we knew that T cells were increased in the brains of people with Alzheimer’s disease and other tauopathies, but we didn’t know for sure that they caused neurodegeneration. These findings open up exciting new therapeutic approaches. Some widely used drugs target T cells. Fingolomid, for example, is commonly used to treat multiple sclerosis, which is an autoimmune disease of the brain and spinal cord. It’s likely that some drugs that act on T cells could be moved into clinical trials for Alzheimer’s disease and other tauopathies if these drugs are protective in animal models.”
Alzheimer’s develops in two main phases. First, plaques of the protein amyloid beta start to form. The plaques can build up for decades without obvious effects on brain health. But eventually, tau also begins to aggregate, signaling the start of the second phase.
From there, the disease quickly worsens: People begin having trouble remembering things and thinking clearly as the brain shrinks, nerve cells die, and neurodegeneration progresses.
Microglia and their role in Alzheimer’s have been intensely studied. The cells become activated and dysfunctional as amyloid plaques build up, and even more so once tau begins to aggregate. Microglial dysfunction worsens neurodegeneration and accelerates the course of the disease.
First author Xiaoying Chen, PhD, an instructor in neurology, wondered about the role of other, less studied immune cells in neurodegeneration. She analyzed immune cells in the brains of mice genetically engineered to mimic different aspects of Alzheimer’s disease in people, looking for changes to the immune cell population that occur over the course of the disease.
Two of the mice strains generate significant amyloid deposits but do not have brain atrophy, mirroring the early stages of the illness in humans. A third strain, representative of the later phase, develops tau tangles, brain atrophy, neurodegeneration and behavioral deficits by 9½ months of age. A fourth mouse strain does not develop amyloid plaques, tau tangles or cognitive impairments; it was studied for comparison.
Along with Chen and Holtzman, the research team included Maxim N. Artyomov, PhD, the Alumni Endowed Professor of Pathology & Immunology, and Jason D. Ulrich, PhD, an associate professor of neurology, among others.
The brains of tau mice had significantly more T cells than the brains of amyloid or control mice, according to the researchers. Remarkably, the regions of the brain with the greatest amount of microglia and degeneration also had the highest number of T cells. In the brains of persons who had died from Alzheimer’s disease, T lymphocytes were equally prevalent at regions of tau accumulation and neurodegeneration.
According to additional animal research, the two types of immune cells collaborate to produce an inflammatory milieu that is conducive to brain injury. Microglia emit molecules that attract and activate T cells from the blood, while T cells release molecules that encourage microglia to become more pro-inflammatory.
Removing either T cells or microglia disrupted the harmful link between them and significantly lessened brain damage. For example, when tau mice were given an antibody to deplete their T cells, they had fewer inflammatory microglia in their brains, less neurodegeneration and atrophy, and an improved ability to perform tasks such as building a nest and remembering recent things.
“What got me very excited was the fact that if you prevent T cells from getting into the brain, it blocks the majority of the neurodegeneration,” Holtzman said.
“Scientists have put a lot of effort into finding therapies that prevent neurodegeneration by affecting tau or microglia. As a community, we haven’t looked at what we can do to T cells to prevent neurodegeneration. This highlights a new area to better understand and therapeutically explore.”