Surface plasmon resonance (SPR) is a phenomenon in which electrons in a thin metal sheet are excited by light directed at a specific angle of incidence and then travel parallel to the sheet. Assuming a constant light source wavelength and a thin metal sheet, the angle of incidence that causes SPR is related to the material’s refractive index, and even a small change in the refractive index will result in SPR not being observed. As a result, SPR can be used to detect specific substances (analytes), and SPR biosensors have been developed to detect a variety of important biomarkers.
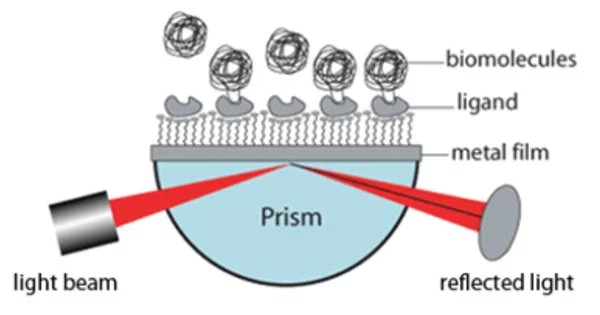
Surface Plasmon Resonance (SPR) is a popular label-free optical technique for real-time analysis of biomolecular interactions. SPR detects changes in refractive index near a thin metal film, typically gold or silver, coated with a thin layer of a recognition molecule, such as an antibody or a receptor. When a ligand molecule binds to a recognition molecule on the metal surface, the refractive index at the interface changes, causing the angle of incident light or the intensity of reflected light to change. This change is then measured and used to determine the interaction’s binding kinetics, which includes the association and dissociation rates, binding affinity, and specificity.
Explanation
The surface plasmon polariton is a non-radiative electromagnetic surface wave that travels parallel to the negative permittivity/dielectric material interface. Because the wave is on the boundary between the conductor and the external medium (air, water, or vacuum, for example), these oscillations are extremely sensitive to changes in this boundary, such as molecule adsorption on the conducting surface.
It is a real-time optical technique for measuring molecular interactions. When plane-polarized light strikes a metal film under total internal reflection conditions, SPR can occur. The refractive index of the medium on the sensor chip directly influences the SPR signal. Biomolecule binding causes changes in the refractive index on the sensor surface.
Applications
SPR has numerous applications, including drug discovery, biomolecular interactions, biotherapeutic development, and quality control. It is a powerful tool for studying protein-protein, protein-small molecule, and nucleic acid interactions, as well as cell-extracellular matrix protein interactions. SPR is a valuable tool for both academic and industrial research because it is highly sensitive, can detect interactions in real-time, and requires only small sample volumes.