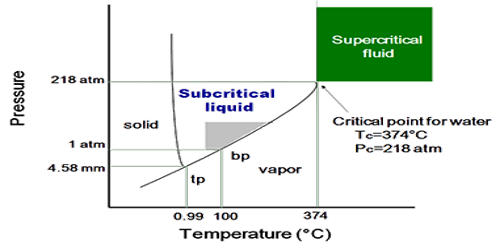
A supercritical fluid (SCF) is one kind of fluid that is highly compressed gases that combine properties of gases and liquids in an intriguing manner. It is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist. It is a substance which can be either liquid or gas, used in a state above the critical temperature and critical pressure where gases and liquids can coexist. It can effuse through solids like a gas, and dissolve materials like a liquid. They have no surface tension because they are not subject to the vapor-liquid boundary so no molecules have the attraction to the interior of the liquid.
A supercritical fluid has both the gaseous property of being able to penetrate anything and the liquid property of being able to dissolve materials into their components. In addition, close to the critical point, small changes in pressure or temperature result in large changes in density, allowing many properties of a supercritical fluid to be “fine-tuned”. They have the low viscosity of gas and the high density of a liquid, making it impossible to liquefy the matter using any amount of pressure. The fluid will also be called supercritical even if its temperature is below critical point value. In that case, it will be a highly compressed liquid. But pressure is mandatory to be above the critical value.
In 1822 Baron Charles Cagniard de la Tour discovered supercritical fluids while conducting experiments involving the discontinuities of the sound of a flint ball in a sealed cannon barrel filled with various fluids at various temperatures (“Charles Cagniard de la Tour”). Supercritical fluids can be applied to remove polluting materials from the environment. The theory and practice of this technology are of increasing interest at the present time.
Supercritical fluids have many interesting properties, especially they have unique solvent properties:
- The liquid-like density promotes the solubility of many substances in supercritical fluids.
- Simultaneously supercritical fluids show significantly small surface tension, allowing for highly efficient and harmless drying conditions.
- Supercritical fluids although being a fluid provide gas-like viscosity and diffusivity.
Supercritical fluids occur in the atmospheres of the gas giants Jupiter and Saturn, and probably in those of the ice giants Uranus and Neptune. In a range of industrial and laboratory processes, they are used as a substitute for organic solvents. can occur in nature. For example, in places like underwater volcanoes, specifically those located deep beneath the ocean’s surface, supercritical water is formed because of the immense pressure due to the depth and the intense heat from the vents of the volcano.
Supercritical fluids are useful in science today for purposes ranging from the extraction of floral fragrance from flowers and the process of creating decaffeinated coffee to applications in food science and functional food ingredients, pharmaceuticals, cosmetics, polymers, powders, bio- and functional materials, nano-systems, natural products, biotechnology, fossil and biofuels, microelectronics and environment. The densities and viscosity of a supercritical fluid are subject to change when pressure or temperature are tampered with, and the supercritical fluid of a substance can have very different properties than the regular fluids. Carbon dioxide and water are the most commonly used supercritical fluids, being used for decaffeination and power generation, respectively.