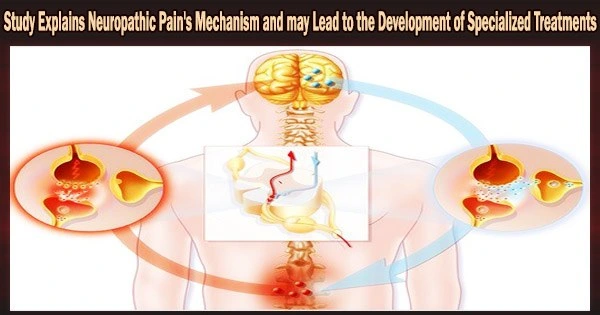
Neuropathic pain is a form of chronic pain that develops from nerve damage brought on mostly by metabolic illnesses like diabetes and arthritis or by the adverse effects of some types of chemotherapy.
Depending on the nation, it is estimated that between 3% and 15% of the population are affected, however there are no known treatments at this time. The medications that are currently prescribed were initially intended to treat different disorders like depression or epilepsy.
But now, after a decade of study, a team of Brazilian researchers has succeeded in describing a mechanism linked to the development of neuropathic pain, launching a new phase of their investigation into the search for medications that can act on the aforementioned metabolic pathway and pointing to a route for the creation of targeted therapies.
The study demonstrated that the presence of dendritic cells in the meninges the membranes that coat the central nervous system plays a role in neuropathic pain through an increase in the metabolic kynurenine pathway.
This pathway plays critical roles in numerous physiological processes, including the formation of the immune response, and is in charge of the amino acid metabolism necessary for the creation of vitamin B3 (tryptophan). Increased kynurenine production has previously been linked, for instance, to the emergence of psychiatric diseases such as depression.
In this study, the scientists found that neuropathic pain was nullified when the kynurenine pathway initiated by the enzyme IDO1 (indoleamine 2,3-dioxygenase 1) was blocked pharmaceutically or genetically. An article reporting the results is published in the Journal of Clinical Investigation.
“It was a very lengthy study because we wanted to explore in depth all the mechanisms that could be involved. It required collaboration in Brazil and abroad with leading experts, such as Andrew Mellor (a professor at Augusta University, formerly Georgia Regents, in the United States), one of the world’s foremost specialists in IDO. The results we obtained offer the prospect of developing novel compounds to block this pathway. We believe inhibitors of the pathway could play a key role in controlling neuropathic pain,” Thiago Mattar Cunha, last author of the article, told Agência FAPESP.
We used various tools to prove and elucidate the role played by these enzymes and other products in neuropathic pain. We used mice as a model and showed that neuropathic pain decreased in animals with a deficiency of these enzymes, or with the enzymes inhibited. We then set out to understand the mechanism.
Thiago Mattar Cunha
According to Mattar Cunha, the discovery has opened up a previously unexplored field of research into the role of the meninges in the production of pain.
Inflammation
The kynurenine pathway, whose synthesis depends on a number of enzymes but principally on IDO1, has previously been demonstrated to be implicated in pain. The researchers postulated that as pro-inflammatory cytokines promote IDO1 during pathological processes, particularly inflammation, the neuroinflammation that results in neuropathic pain would also boost IDO, increasing the amounts of these neurotoxic or neurostimulatory metabolites.
“We used various tools to prove and elucidate the role played by these enzymes and other products in neuropathic pain,” Mattar Cunha said. “We used mice as a model and showed that neuropathic pain decreased in animals with a deficiency of these enzymes, or with the enzymes inhibited. We then set out to understand the mechanism.”
In the article, the researchers explain that they used “a well-established model of peripheral nerve injury-induced neuropathic pain,” known as the spared nerve injury model, and activation of spinal cord microglial cells (a type of central nervous system cell involved in the immune response). In the spinal cord, kynurenine was metabolized by astrocytes, and the glutamatergic receptor was activated.
“We showed for the first time that when peripheral nerves are injured, immune cells infiltrate the meninges that protect the spinal cord and dorsal root ganglion, producing mediators which cause or maintain pain hypersensitivity. IDO1 and its metabolites are produced in this process. We showed that these metabolites come mainly from dendritic cells in the meninges, causing hypersensitivity and amplifying the glutamatergic pathway, whose receptors play an important role in chronic pain,” Mattar Cunha said.
Although the study used pain models involving physical nerve injury, he added, the mechanism is similar in other neuropathic conditions, such as infectious diseases caused by HIV and other viruses.
In order to work together on investigations of compounds and medications that can target the process and decrease neuropathic pain, the researchers are currently looking for partnerships with pharmaceutical companies or other research facilities.
An article published in Dec. 2021 Nature Neuroscience by CRID scientists, including Mattar Cunha, and researchers at Harvard University in the U.S. showed that a non-lethal component of anthrax toxin has high analgesic power and can act directly on pain-signaling neurons.
Bacillus anthracis, a bacterium whose spores can cause skin lesions and respiratory issues leading to death in a few hours, produces the anthrax toxin. The component can be utilized as a carrier vehicle to transfer other pain-blocking compounds into nerve cells since it binds to a receptor in pain-sensing neurons, according to the researchers.