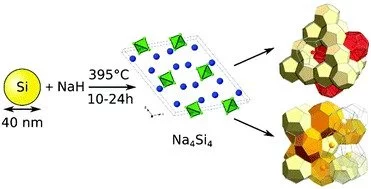
Sodium silicide is a chemical compound made up of sodium (Na) and silicon (Si). It is a binary inorganic compound consisting of sodium and silicon. It is a solid black or grey crystalline material. It is an inorganic material from the silicide family. Under controlled conditions, silicon can be reacted with sodium metal to produce sodium silicide. The resulting compound is a gray or black solid with the chemical formula NaSi.
The high reactivity of sodium silicide with water and moisture is well known. When exposed to water, it undergoes a vigorous reaction, emitting hydrogen gas (H2) and forming sodium hydroxide (NaOH) and silicic acid (H4SiO4). This reaction is highly exothermic and, under certain conditions, can be explosive.
Structure
Sodium silicide has the chemical formula NaSi. It typically exists as a gray or dark gray crystalline solid. The crystal structure of sodium silicide is composed of Si atoms arranged in a cubic close-packed lattice, with sodium atoms occupying the interstitial sites.
Reaction
Sodium silicide is highly reactive, particularly when exposed to water or moisture. It reacts vigorously with water to produce sodium hydroxide (NaOH) and hydrogen gas (H2). This reaction is exothermic and can be potentially dangerous due to the release of flammable hydrogen gas.
Sodium silicide reacts readily with water yielding gaseous hydrogen and aqueous sodium silicate in an exothermic reaction (~175 kJ·mol−1):
2 NaSi + 5 H2O → 5 H2 + Na2Si2O5
This is used in hydrogen technologies to generate hydrogen as a fuel. And is also used as high energy dense storage for hydrogen under low pressure.
Because of its water reactivity, sodium silicide is primarily used as a reducing agent in a variety of chemical reactions. It can be used to purify metals by reducing metal oxides or other compounds. Sodium silicide can also be used in pyrotechnics and as a hydrogen gas source.
Uses
Sodium silicide is primarily used as a reducing agent in various chemical reactions. It is often employed in metallurgy and the production of certain alloys. Additionally, sodium silicide has been investigated for its potential applications in hydrogen storage, as it can release hydrogen gas upon reaction with water.
Safety
However, it is important to note that sodium silicide is a hazardous material that must be handled with caution. It should be kept away from moisture and incompatible materials. When working with sodium silicide, take the necessary precautions, such as wearing protective clothing, gloves, and eyewear, and working in a well-ventilated area.
Sodium silicide should be handled with caution due to its reactivity with moisture and water. It can cause burns or irritation upon contact with skin or eyes. When working with sodium silicide, appropriate safety measures, such as wearing protective clothing and goggles, should be followed.