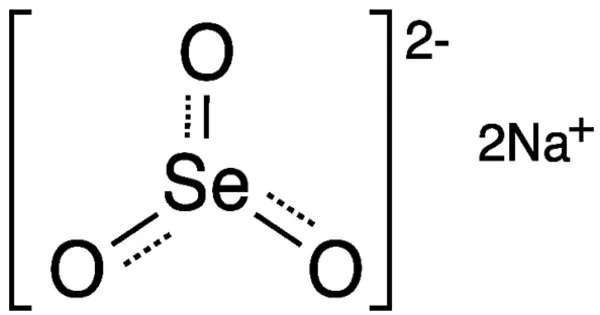
Sodium selenite is the inorganic compound with the formula Na2SeO3. This salt is a colourless solid. The pentahydrate Na2SeO3(H2O)5 is the most common water-soluble selenium compound. It is a white crystalline solid that is commonly used as a source of selenium in various industrial applications and as a dietary supplement.
It is a sodium salt of selenous acid, and it contains the essential trace element selenium. Selenium plays a crucial role in several biological processes, including the function of enzymes and antioxidants in the body.
Properties
Sodium selenite is usually found as a white crystalline powder or colorless crystals. It is soluble in water, with solubility increasing as temperature rises. It is toxic and should be handled with caution. It can be harmful if ingested, inhaled, or absorbed through the skin. It is used in scientific research and laboratory experiments related to selenium compounds and their properties.
- Chemical formula: Na2O3Se
- Molar mass: 172.948 g·mol−1
- Appearance: colourless solid
- Density: 3.1 g/cm3
- Melting point: decomposes at 710 °C
- Solubility in water: 85 g/100 mL (20 °C)
- Solubility: insoluble in ethanol
- Crystal structure: tetragonal
Applications
Together with the related barium and zinc selenites, sodium selenite is mainly used in the manufacture of colorless glass. The pink color imparted by these selenites cancels out the green color imparted by iron impurities.
Because selenium is an essential element, sodium selenite is an ingredient in dietary supplements such as multi-vitamin/mineral products, but supplements that provide only selenium use L-selenomethionine or a selenium-enriched yeast.
Safety
Selenium is toxic in high concentrations. As sodium selenite, the chronic toxic dose for human beings was described as about 2.4 to 3 milligrams of selenium per day. In 2000, the U.S. Institute of Medicine set the adult Tolerable upper intake levels (UL) for selenium from all sources – food, drinking water and dietary supplements – at 400 μg/day.