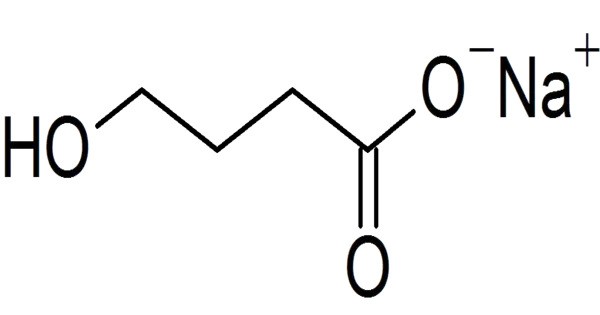
Sodium oxybate is the sodium salt of gamma-hydroxybutyrate (GHB), a naturally occurring neurotransmitter. It sold under the brand name Xyrem among others, is a medication used to treat symptoms of narcolepsy: sudden muscle weakness and excessive daytime sleepiness. It is used sometimes in France and Italy as an anesthetic given intravenously. Administered orally as a liquid, it’s typically taken in two nightly doses due to its short half-life of about 30-60 minutes.
Sodium oxybate is the sodium salt of γ-hydroxybutyric acid (GHB). The clinical trials for narcolepsy were conducted just as abuse of GHB as a club drug and date rape drug became a matter of public concern. It works by enhancing slow-wave sleep and modulating GABA and dopamine pathways, though its exact mechanism isn’t fully understood.
Properties
- Molecular Weight: 126.09 g/mol.
- Appearance: White to off-white crystalline powder; hygroscopic.
- Solubility: Highly soluble in water, soluble in methanol, and slightly soluble in ethanol; insoluble in most organic solvents.
- pH: Aqueous solutions are alkaline (pH ~7.5–9.0).
- Pharmacological Properties: Central nervous system depressant. It acts as a neuromodulator, primarily affecting GABA-B receptors and GHB-specific receptors, influencing dopamine release and sleep regulation.
- Stability: Stable under controlled conditions but sensitive to moisture and extreme pH. Must be stored in airtight containers.
- Boiling/Melting Point: As a salt, it decomposes before melting; GHB (free acid) has a boiling point of ~230°C.
Medical uses
Clinical use of sodium oxybate was introduced in Europe in 1964 as an anesthetic given intravenously, but it was not widely used since it sometimes caused seizures. As of 2006, it was still authorized for this use in France and Italy but not widely used.
The major use of sodium oxybate is in treating two of the symptoms of narcolepsy – cataplexy (sudden muscle weakness) and excessive daytime sleepiness.
Approved by the FDA in 2002, it’s tightly regulated due to its potential for abuse and dependence, classified as a Schedule III controlled substance in the U.S. Common side effects include nausea, dizziness, headache, and sleepiness, while serious risks involve respiratory depression, coma, or death, especially if mixed with alcohol or other depressants. It’s contraindicated in patients with succinic semialdehyde dehydrogenase deficiency or those using sedatives like benzodiazepines.
Occurrences
- Natural Occurrence: GHB, the parent compound, occurs naturally in trace amounts in the human brain and other mammalian tissues as a metabolite of GABA (gamma-aminobutyric acid). It is also found in small quantities in some foods and fermented beverages (e.g., wine, in trace amounts).
- Synthetic Production: Sodium oxybate is synthetically produced for medical use. It is not found naturally in significant quantities and is manufactured via controlled chemical processes, typically involving the neutralization of GHB with sodium hydroxide.
- Medical Use: Sodium oxybate is used therapeutically (e.g., Xyrem) to treat narcolepsy symptoms like cataplexy and excessive daytime sleepiness. It is a Schedule III controlled substance in the U.S. due to its potential for abuse.
Safety
Sodium oxybate has a narrow therapeutic index, requiring precise dosing. Overdose can lead to respiratory depression, coma, or death. Its production, distribution, and use are tightly controlled globally due to abuse potential.