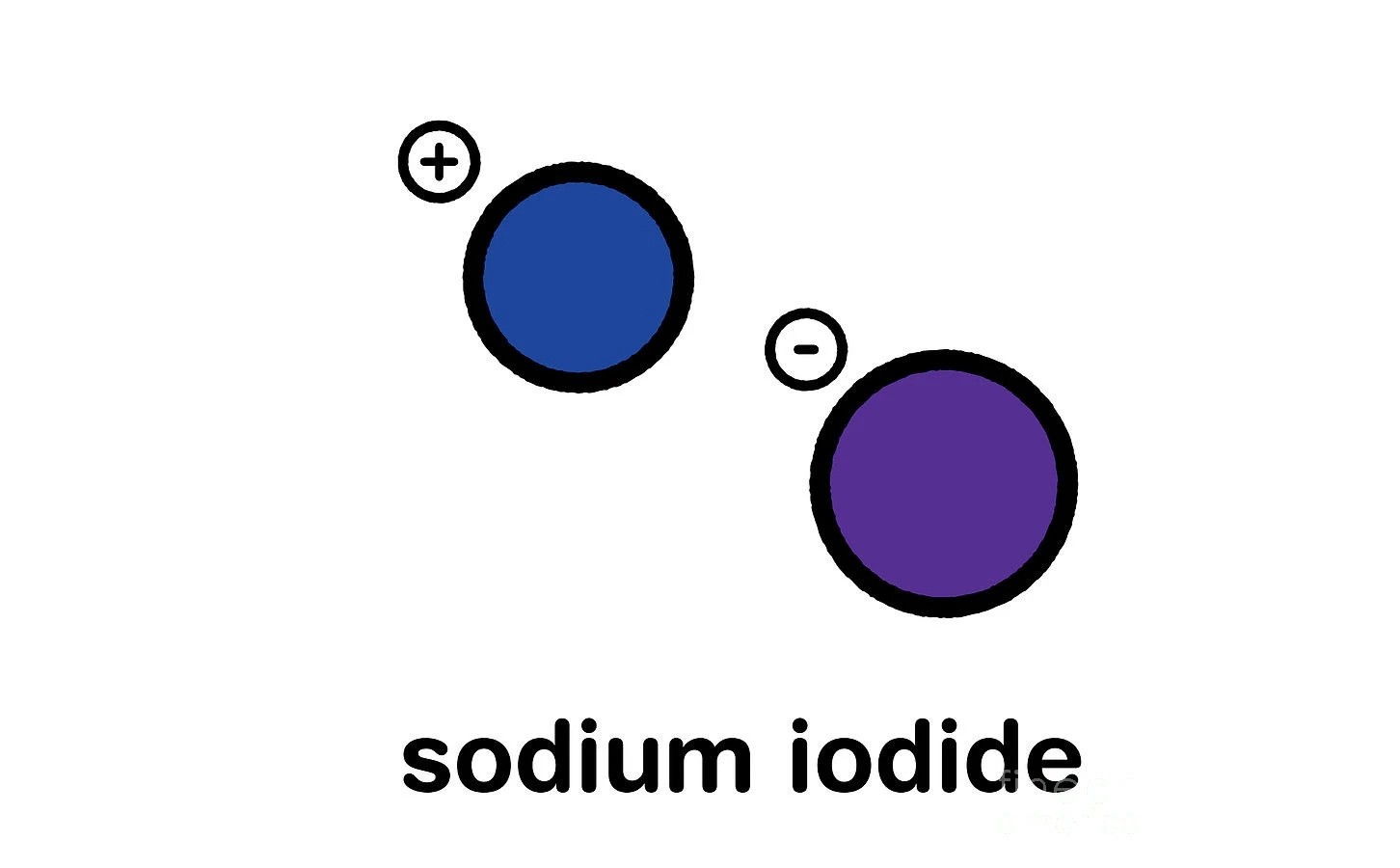
Sodium iodide (chemical formula NaI) is an ionic compound formed from the chemical reaction of sodium metal and iodine. It is a white, crystalline solid that is highly soluble in water. Under standard conditions, it is a white, water-soluble solid comprising a 1:1 mix of sodium cations (Na+) and iodide anions (I−) in a crystal lattice. It is used mainly as a nutritional supplement and in organic chemistry. It is produced industrially as the salt formed when acidic iodides react with sodium hydroxide. It is a chaotropic salt.
Stability
Iodides (including sodium iodide) are detectably oxidized by atmospheric oxygen (O2) to molecular iodine (I2). I2 and I− complex to form the triiodide complex, which has a yellow color, unlike the white color of sodium iodide. Water accelerates the oxidation process, and iodide can also produce I2 by photooxidation, therefore for maximum stability sodium iodide should be stored under dark, low temperature, low humidity conditions.
Properties
- Chemical formula: NaI
- Molar mass: 149.894
- Appearance: white solid deliquescent
- Odor: odorless
- Density: 3.67 g cm−3
- Melting point: 661 °C (1,222 °F; 934 K)
- Boiling point: 1,304 °C (2,379 °F; 1,577 K)
- Solubility in water: 1587 g/L (0 °C), 3020 g/L (100 °C)
- Solubility: ethanol, acetone
Occurrences
- Natural Sources: Sodium iodide is not commonly found in nature as a mineral. Instead, iodine is typically found in marine environments, such as seawater, and in some minerals.
- Production: It can be synthesized through the neutralization of sodium hydroxide with hydroiodic acid.
Applications
- Medical Imaging: It is used in nuclear medicine as a radioactive tracer for thyroid imaging. Iodine is essential for thyroid hormone production.
- Reagents in Chemistry: It is often used in organic synthesis and as a source of iodide ions in various reactions.
- Antiseptics: Iodine has antiseptic properties, and sodium iodide can be used in some medical preparations.