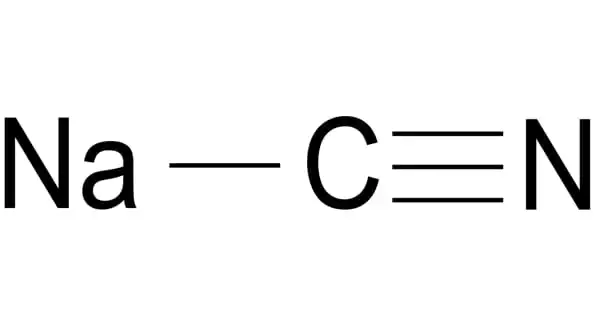Sodium cyanide, abbreviated NaCN, is a poisonous compound. It is a cyanide salt with an equal amount of sodium cations and cyanide anions. It is a water-soluble white solid. Cyanide has a high affinity for metals, which contributes to its toxicity. It acts as an inhibitor of EC 1.15.1.1 (superoxide dismutase). It is a sodium salt, a cyanide salt, and a one-carbon compound.
Sodium cyanide solution is a colorless, clear aqueous solution. Its primary application, gold mining, also takes advantage of its high reactivity to metals. It has a moderately strong foundation. When exposed to acid, it produces the toxic gas hydrogen cyanide:
NaCN + H2SO4 → HCN + NaHSO4
Production and chemical properties
Sodium cyanide is produced by treating hydrogen cyanide with sodium hydroxide:
HCN + NaOH → NaCN + H2O
In 2006, global production was estimated to be 500,000 tons. Previously, it was made using the Castner process, which involved the reaction of sodium amide with carbon at high temperatures.
NaNH2 + C → NaCN + H2

Solid NaCN has a structure similar to sodium chloride. Each anion and cation has a six-coordinate. KCN (potassium cyanide) has a similar structure.
Because the salt is derived from a weak acid, hydrolysis easily converts sodium cyanide to HCN; the moist solid emits small amounts of hydrogen cyanide, which is thought to smell like bitter almonds (not everyone can smell it—the ability is due to a genetic trait). When sodium cyanide reacts quickly with strong acids, it produces hydrogen cyanide. This hazardous process poses a significant risk in the presence of cyanide salts. It is detoxified most efficiently with hydrogen peroxide (H2O2) to produce sodium cyanate (NaOCN) and water:
NaCN + H2 2 → NaOCN + H2O
Uses
Commercially, sodium cyanide is used for fumigation, electroplating, extracting gold and silver from ores, and chemical manufacturing.
Sodium cyanide is widely used in industries around the world, most notably in gold mining. Although most of us imagine a 19th-century gold miner panning for nuggets, this is not the industrial method used today.
Toxicity
Sodium cyanide produces hydrogen cyanide gas, a highly toxic chemical asphyxiant that impairs the body’s ability to use oxygen. Sodium cyanide poisoning can be fatal in a matter of minutes. It has systemic (whole-body) effects, especially on the organ systems most sensitive to low oxygen levels: the central nervous system (brain), the cardiovascular system (heart and blood vessels), and the pulmonary system (lungs).
Although there are risks associated with skin absorption, the most serious risk is ingestion. Inhaling or swallowing sodium cyanide disrupts oxygen transport, resulting in serious medical problems and, eventually, death.