A new study has revealed how HIV makes its way into the nucleus when it invades a cell. Because viruses must steal someone else’s cell in order to proliferate, they’ve honed their skills, devising a variety of techniques.
New research by two University of Chicago scientists reveals how HIV squirms its way into the nucleus when it invades a cell. According to their models, the HIV capsid, which is cone-shaped, inserts its smaller end into the nucleus’ pores before ratcheting itself in. Once the pore is sufficiently open, the capsid is elastic enough to pass through. Importantly, the researchers stated that both the structural flexibility of the capsid and the pore itself have a role in the infiltration process.
The discovery, made through a simulation of thousands of proteins interacting, will lead to a better understanding of HIV and new targets for therapeutic medications. “For example, you could try to make the HIV capsid less elastic, which our data suggests would limit its ability to enter the nucleus,” said Arpa Hudait, a research scientist at UChicago and the paper’s first author.
The work also gives the most detailed simulation of the nuclear pore, which is involved in numerous biological processes.
The pore complex is an incredible piece of machinery; it can’t let just anything into the nucleus of your cell, or you’d be in real trouble, but it’s got to let quite a bit of stuff in. And somehow, the HIV capsid has figured out how to sneak in.
Gregory Voth
Capsid vs. cell
Hudait is a member of the laboratory of Gregory Voth, the Haig P. Papazian Distinguished Service Professor of Chemistry, which specializes in simulations to unravel the complex biological processes that occur as viruses attack a cell.
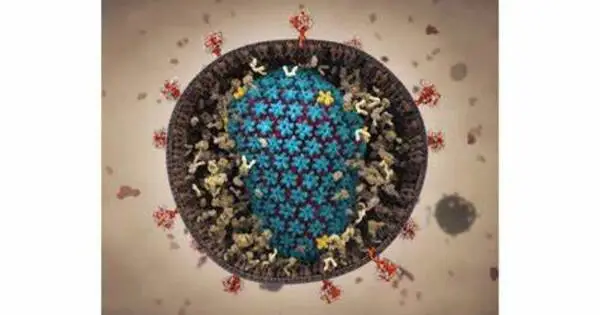
In this case, Voth and Hudait focused on what’s known as the HIV capsid – the capsule containing HIV’s genetic material, which enters a host cell’s nucleus and forces the cell to make copies of the key HIV components.
The capsid is a complex piece of machinery, made of more than a thousand proteins assembled into a cone-like shape, with a smaller and larger end. To get into the host cell’s nucleus, it must sneak in. But scientists didn’t know exactly how this happens. “This part has been a mystery for years,” said Voth, the senior author on the paper. “For a long time, no one was sure whether the capsid broke apart before entering the pore or afterward, for example.”
Recent imaging studies have suggested the capsid stays intact wriggling through the nuclear pore complex. This is essentially the mail slot where the nucleus sends and receives deliveries.
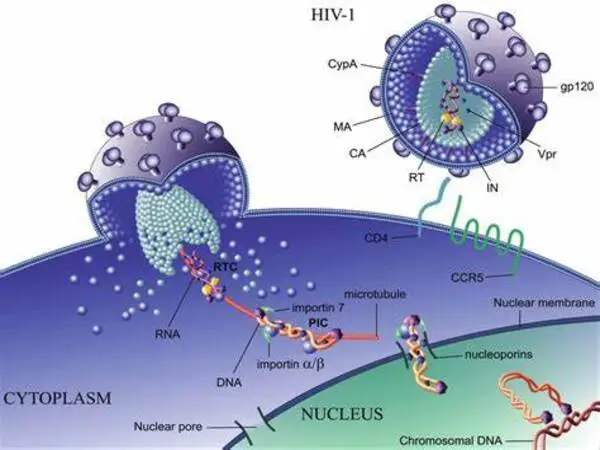
“The pore complex is an incredible piece of machinery; it can’t let just anything into the nucleus of your cell, or you’d be in real trouble, but it’s got to let quite a bit of stuff in. And somehow, the HIV capsid has figured out how to sneak in,” Voth said. “The problem is, we can’t watch it live. You have to go to heroic experimental efforts to even get a single, moment-in-time snapshot.”
To fill up the gaps, Hudait created a meticulous computer simulation of both the HIV capsid and the nuclear pore complex, which included thousands of proteins functioning together. Running the simulations, the scientists discovered that it was far easier for the capsid to enter the pore by inserting its tiniest end first and then gradually ratcheting itself in. “It doesn’t need active work to do it, it’s just physics — what we call an electrostatic ratchet,” Voth explained. “It’s kind of like if you’ve ever had a seatbelt tighten up on you, where it just keeps getting tighter and tighter.”
They also discovered that the pore and the capsid deform as it moves. Interestingly, the lattice of molecules that comprise the capsid structure generates little patches of less order to handle the tension of the pressure. “It’s not like a solid compressing or expanding, as one might have expected,” Hudait went on to say.
The discovery may help explain why capsids are cone-shaped rather than cylinder-shaped, which may appear to be simpler to slip through a pore. According to the experts, each aspect of HIV’s path through the body provides an opportunity to identify vulnerabilities that medications can be developed to target. It also provides a broader perspective on a fundamental feature of life.
“I think this modeling also gives us a new way to understand how many things get into the nucleus, not just HIV,” said Voth.