Researchers from UCL, Great Ormond Street Hospital, and the Wellcome Sanger Institute have revealed new results that explain why some children experience prolonged remission after receiving cutting-edge CAR T-cell therapy for leukemia.
The collaborative research endeavor, which was just reported in Nature Medicine, combines cutting-edge immune therapy design knowledge with cutting-edge computational analysis to find a genetic profile of CAR T-cells that will be the most effective in the long run.
CAR T-cells, which are genetically altered T-cells (a type of immune cell) meant to target leukemia, have become an established therapy option for children with relapsed or incurable rare forms of (B-cell acute lymphoblastic or B ALL) in recent years.
The duration of the CAR T-cells in the body is an important component in determining whether the treatment will result in long-term remission of leukemia, allowing children to live cancer-free. Until today, little was understood about what causes these cells to survive in the body and, as a result, if the treatment is likely to work long-term without additional treatments.
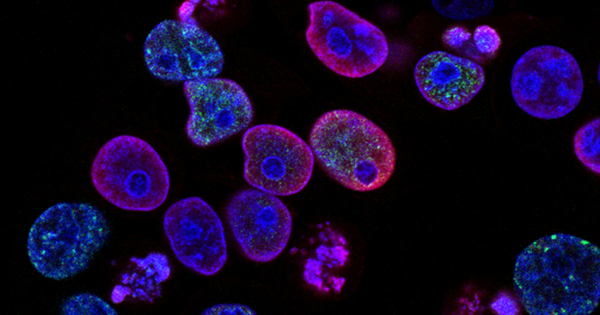
As part of the CARPALL study, a collaborative research team from Great Ormond Street Hospital (GOSH), the Wellcome Sanger Institute, and the UCL Great Ormond Street Institute of Child Health (UCL GOS ICH) worked with families for years after their CAR T-cell treatment (called AUTO1) to begin to piece together why some CAR T-cells stay in the body long-term.
This study is the first step toward understanding why some CAR T-cells persist. The team intends to expand on the signature revealed in this experiment to find crucial indicators in cell populations and, ultimately, determine whether there is a mechanism to detect, or perhaps produce, CAR T-cells that will survive long-term before therapy begins.
“Through cutting-edge single-cell genomics, we have been able to crack the code of persistence in CAR T-cells in children for the first time,” stated Dr. Nathaniel Anderson, principal author and Marie Sklodowska-Curie Fellow at the Wellcome Sanger Institute.
“We hope that our research will provide the first clue as to why some CAR T-cells survive for so long – which we know is critical for keeping children cancer-free after treatment.” Finally, this research will assist us to improve an already life-changing treatment.”
The objective is that this knowledge will eventually allow clinical teams administering CAR T-cell therapies to better predict which patients would respond best to treatments and allow manufacturers to enhance their processes to support persistence, resulting in better patient outcomes.
“This data for the first time shows us the characteristics of long-lasting CAR T-cells which are responsible not only for curing children with ALL in our study but also seen in adults treated with a different CAR T-cell product for a different type of leukemia,” said Dr. Sara Ghorashian, co-senior author, Consultant in Paediatric Haematology at GOSH, and Honorary Senior Clinical Lecturer at the UCL GOS ICH. As a result, we have confidence that the signature will reveal mechanisms of CAR T-cell persistence in general, allowing us to build better treatments.
“We are grateful to all of the children and families who make research like ours possible; it is only because of their dedication that we are able to increase our understanding of these new therapies and develop better treatments for children all over the world.”
Studying CAR T-cells in-depth: The researchers were able to investigate cells from ten children who participated in a groundbreaking clinical trial (the CARPALL trial) for up to five years following their initial CAR T-cell treatment. This has given them a fresh insight into why some of these CAR T-cells remain in a patient’s bloodstream while others vanish, allowing the cancer to recur in some situations.
The scientists were able to establish a distinctive “signature” in long-lasting CAR T-cells by using approaches that analyze individual cells at a genomic level to determine what they do. The signature suggested that long-lasting CAR T-cells in the blood undergo a transformation, allowing them to continue policing the patient’s body for cancer cells.
This characteristic was found in cells, patients, and adults treated with a different CAR T-cell product for a different form of leukemia. However, it was not found in other types of immune cells. This revealed that the signature discovered by the scientists may not only be a marker of these long-lasting cells but may also be what permits them to survive in the body and allow for a prolonged remission in children.
The researchers identified crucial genes in CAR T-cells that appeared to allow them to remain in the body for an extended period of time as part of the study. Importantly, these genes will serve as a starting point for future research into identifying markers of persistence in CAR T-cell products as they are manufactured, with the goal of ultimately improving their effectiveness.
“This study is a fantastic step forward in our understanding of CAR T-cell persistence and illustrates the power of collaborative science and combining pioneering clinical research with cutting-edge genomic science,” said Dr. Sam Behjati, co-senior author, Group Lead and Wellcome Senior Research Fellow at the Wellcome Sanger Institute and Honorary Consultant Paediatric Oncologist at Addenbrooke’s Hospital, Cambridge. It is critical that we continue to develop and improve these novel medicines in order to benefit more children with leukemia worldwide.”
The dedication of research families: Such studies are only possible because of the dedication of the children and families that participate in the research. Children had to continue donating cells to the study for up to five years after their initial treatment in order for investigators to evaluate the long-term persistence of cells.
Austin was diagnosed with B ALL when he was two years old, and by the age of eight, he’d had three relapses and significant therapy, including two bone marrow transplants. He had exhausted all conventional therapy choices by the time of his fourth relapse. As part of the CARPALL clinical trial, Austin received an injection of CAR T-cells in October 2016.
Austin, now 14, is still cancer-free six years later, with long-lasting CAR T-cells detected in his blood. He is one of ten children who have given samples to this study since their infusions. “It’s not an exaggeration to say that Austin would not be alive if it weren’t for research,” his father Scott stated. We wanted to offer something back to the GOSH research teams that had given us so much. Participating in this study not only provides us with that opportunity, but we also hope that Austin’s data can benefit other families like ours in the future.
“We actually enjoy returning to GOSH to see the team and keep them in our lives.” “I’m so glad Austin was a part of this research journey.”
This ongoing dedication to research is assisting researchers in better understanding new, cutting-edge therapies and improving them for future families.
“We know that immunotherapies like CAR T-cell therapy have seen some great success over the years,” said Dr. Henry Stennett, Research Information Manager at Cancer Research UK, which helped fund the study. “But they don’t work in all patients, and we need to keep working to figure out why.” Such research is critical in moving us closer to making immunotherapies more effective for more cancer patients.”
Reference: “Transcriptional signatures associated with persisting CD19 CAR-T cells in children with leukemia” by Nathaniel D. Anderson, Jack Birch, Theo Accogli, Ignacio Criado, Eleonora Khabirova, Conor Parks, Yvette Wood, Matthew D. Young, Tarryn Porter, Rachel Richardson, Sarah J. Albon, Bilyana Popova, Andre Lopes, Robert Wynn, Rachael Hough, Satyen H. Gohil, Martin Pule, Persis J. Amrolia, Sam Behjati and Sara Ghorashian, 6 July 2023, Nature Medicine.
DOI: 10.1038/s41591-023-02415-3
The INCAR consortium received funding from the CRUK/AIRC Accelerator Award Scheme. Wellcome supplied institutional and individual (SB) financing. NA was supported by Marie Sklodowska-Curie Actions. This work was also supported by the Olivia Hodson Cancer Fund.
Children with Cancer, GOSH Children’s Charity, and the JP Moulton Trust supported the original CARPALL study. The National Institute for Health and Care Research Biomedical Research Centres at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London Hospital, as well as King’s Health Partners, Great Ormond Street Hospital, and University College London Hospital, all provided assistance.