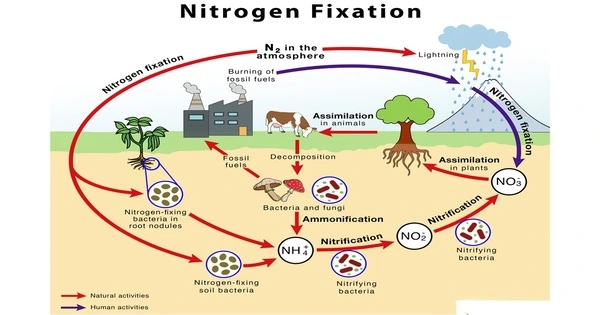
Nitrogen fixation is an important biological process that turns atmospheric nitrogen gas (N2) into a form usable by plants and other organisms. It is a chemical process that converts molecular nitrogen (N2), which has a strong triple covalent bond, into ammonia (NH3) or other nitrogenous chemicals, primarily in soil or aquatic environments but also in industry. Nitrogen gas accounts for approximately 78% of the Earth’s atmosphere, although most plants and animals cannot use atmospheric nitrogen directly.
Nitrogen fixation is required to provide a supply of nitrogen for incorporation into life-sustaining compounds such as proteins and nucleic acids. The nitrogen in the air is molecular dinitrogen, a generally nonreactive chemical that all but a few microbes cannot exploit for metabolism. Biological nitrogen fixation, also known as diazotrophy, is an essential microbe-mediated activity that transforms dinitrogen (N2) gas to ammonia (NH3) via the nitrogenase protein complex (Nif).
There are two primary mechanisms of nitrogen fixation:
- Biological Nitrogen Fixation (BNF): This process involves certain microorganisms, mainly bacteria and archaea, that have the ability to convert atmospheric nitrogen into ammonia or other nitrogen compounds. These nitrogen-fixing microorganisms form symbiotic relationships with plants, living in nodules on the roots of leguminous plants (such as peas, beans, and clover) or as free-living organisms in the soil. Some well-known nitrogen-fixing bacteria include Rhizobium and Bradyrhizobium.
- Industrial Nitrogen Fixation: This process involves the Haber-Bosch process, an industrial method developed in the early 20th century. This process uses high pressure and high temperature to convert atmospheric nitrogen and hydrogen gas into ammonia (NH3), which can be further processed to produce fertilizers and other nitrogen-containing compounds. The industrial nitrogen fixation process has had a profound impact on agriculture, significantly increasing crop yields and supporting the global food supply.
Importance
Nitrogen fixation is critical to life because fixed inorganic nitrogen molecules are necessary for the production of all nitrogen-containing organic compounds, including amino acids, proteins, nucleoside triphosphates, and nucleic acids. Agriculture and fertilizer production rely on it as part of the nitrogen cycle. It is also indirectly related to the production of all nitrogen chemical compounds, including some explosives, medicines, and colors.
Nitrogen fixation is critical for nutrient cycle and ecosystem dynamics. It is an essential component of the nitrogen cycle, which includes processes like ammonification, nitrification, denitrification, and absorption. Nitrogen availability affects plant growth, which in turn determines the structure and function of ecosystems. Overall, it is essential for sustaining life on Earth by ensuring a continuous supply of nitrogen in a form that can be utilized by living organisms.