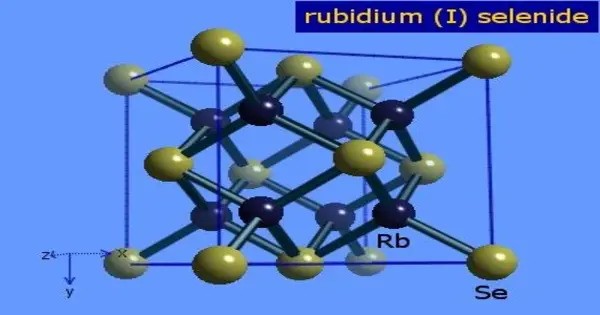
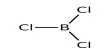
Rubidium selenide is an inorganic compound composed of selenium and rubidium. It belongs to the family of alkali metal selenides, where rubidium acts as a monovalent cation (Rb⁺) and selenium as a divalent anion (Se²⁻). It is a selenide with a chemical formula of Rb2Se. Rubidium selenide is used together with caesium selenide in photovoltaic cells. It forms when rubidium reacts directly with selenium under controlled conditions.
Rubidium selenide has limited but notable applications in materials science, solid-state chemistry, and optoelectronic research, where alkali selenides are explored for their electronic, optical, and semiconducting properties. However, due to its toxicity and reactivity, careful handling is essential.
Properties
Rubidium selenide is an ionic compound, forming a crystal lattice structure that is similar to other alkali chalcogenides such as rubidium sulfide or rubidium telluride. The compound generally appears as a crystalline solid and, like other alkali selenides, it is highly reactive and sensitive to moisture. It is soluble in water, producing strongly alkaline solutions due to the hydrolysis of the selenide ion, which can generate hydrogen selenide gas (H₂Se), a highly toxic and foul-smelling compound.
- Chemical formula: Rb2Se
- Molar mass: 249.89
- Appearance: colourless, highly hygroscopic crystals
- Density: 2.912 g/cm3, 3.16 g/cm3
- Melting point: 733 °C
- Solubility in water: hydrolyses
- Solubility in other solvents: soluble in ethanol and glycerin
Preparation
In terms of preparation, rubidium selenide can be synthesized by direct combination of elemental rubidium and selenium under controlled conditions, often requiring an inert atmosphere to prevent unwanted reactions. It is also obtainable through high-temperature reactions involving rubidium carbonate or rubidium hydroxide with hydrogen selenide.
Rubidium selenide can be prepared by reacting mercury selenide and metallic rubidium. The elements can be synthesized in liquid ammonia. Hydrogen selenide can also be dissolved in an aqueous solution of rubidium hydroxide to eventually form rubidium selenide. This method is similar to the method for preparing rubidium sulfide, because they are both chalcogenide compounds.
RbOH + H2Se → RbHSe + H2O
RbHSe + RbOH → Rb2Se + H2O
Occurrences
Rubidium selenide does not occur naturally in mineral form, as rubidium itself is a rare alkali metal found in small quantities in minerals like lepidolite, pollucite, and carnallite. Selenium is typically obtained as a byproduct of copper refining. Rubidium selenide is therefore produced synthetically in laboratories or specialized facilities rather than mined from natural deposits. Its uses are primarily in research, particularly in materials science, solid-state chemistry, and the study of chalcogenide semiconductors.