Acid hydrolysis is a chemical reaction that involves the use of an acid to break down a chemical compound into its constituent parts through the addition of water molecules. During the process of acid hydrolysis, the acid reacts with the water to produce hydronium ions (H3O+), which attack and break the bonds within the compound, resulting in the formation of smaller molecules or ions.
Extracts from plants and animals have been used as food and traditional medicine by cultures all over the world. For example, Asians, particularly those in Korea, China, and Japan, have utilized sea cucumber extracts to treat cancer, impotence, arthritis, and frequent urination.
While it is simple to dismiss these conventional treatments, sea cucumbers and a number of other marine creatures may hold the key to developing novel drugs.
A class of compounds called “sulfated fucans” (SFs), essentially fucose-rich sulfated polysaccharides found in sea cucumbers and sea urchins, are renowned for their anticoagulant, antiviral, and anticancer properties.
Sulfated fucans have a wide range of biological activities, including anticoagulant, antitumor, and immunomodulatory properties. They are also being investigated for their potential as therapeutic agents for a variety of diseases, including cancer, viral infections, and inflammatory disorders. Recently, they have been investigated for their potency against the SARS-CoV-2 virus.
It is necessary to lower the molecular weight of these SFs by dissolving them into oligosaccharides in order to examine them. A technique known as “mild acid hydrolysis” is frequently used for this. It is crucial to understand the structural alterations that this mild acid hydrolysis has had on SFs.
The phenomenon of acid hydrolysis is constantly emphasized for the depolymerization of sulfated fucans. Our study shows that selective 2-desulfation is a common and expected phenomenon in oligosaccharide production by mild acid hydrolysis of SFs. These results will help further the research on the medicinal properties of SFs, and could potentially result in new medicines for a wide variety of illnesses.
Professor Seon Beom Kim
This is where a team of researchers led by Professor Seon Beom Kim from Pusan National University in Korea and Assistant Professor Vitor H. Pomin from the University of Mississippi, U.S. came in.
The isolation and characterization of sulfated fucans with specific structures is an important area of research for understanding their biological functions and developing new therapeutic applications.
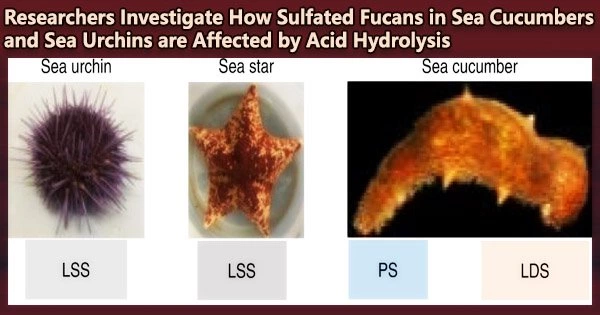
In a recent study published in Carbohydrate Polymers, they studied the mild acid hydrolysis of SFs extracted from two sea cucumber species, Isostichopus badionotus and Holothuria floridana, and one sea urchin species, Lytechinus variegatus, to see the effects of this process during oligosaccharide production.
The study involved contributions from Dr. Marwa Farrag, Dr. Sushil K. Mishra, Dr. Sandeep K. Mishra, Dr. Joshua S. Sharp, and Dr. Robert J. Doerksen, all of them collaborating with Dr. Pomin’s group.
Speaking about the motivation behind their study, Prof. Kim explains, “One of the keys to being an antiviral agent without showing other biological activities is controlling the molecular weight of the polysaccharide. However, specific enzymes that can depolymerize marine polysaccharides are not widely known. As a result, the mild acid hydrolysis route is often the way to go. Therefore, there is an urgent need for physiochemical studies of SFs during the chemical hydrolysis process.”
After the SFs were extracted, the team described each one’s structure, which showed that they are made up of lengthy chains of repeating blocks of four sugars that contain sulfate (SO42-) ions.
Thus, they were classified as 3-linked tetrasaccharide-repeating SFs. The oligosaccharide generated when these SFs were treated with moderate sulfuric acid was then examined to determine the alterations brought on by the hydrolysis.
The scientists discovered that all three SFs exhibited selective 2-desulfation, in which the sulfate ion linked to the second sugar in the repeating tetrasaccharide was removed. This caused the long chains to break up and produce an 8-sugar-long oligosaccharide.
“The phenomenon of acid hydrolysis is constantly emphasized for the depolymerization of sulfated fucans. Our study shows that selective 2-desulfation is a common and expected phenomenon in oligosaccharide production by mild acid hydrolysis of SFs,” says Prof. Kim. “These results will help further the research on the medicinal properties of SFs, and could potentially result in new medicines for a wide variety of illnesses.”