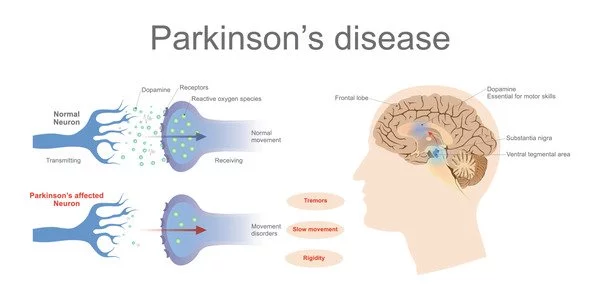
A key finding sheds light on the underlying mechanics of Parkinson’s disease, paving the way for future new treatments. Until recently, our understanding of Parkinson’s disease was relatively restricted, which was reflected in the devastating condition’s limited therapy options and care.
Our current understanding has centered mostly on the genetic elements responsible for familial cases, leaving the vast majority of patients’ causal factors unclear. Researchers from the University of Copenhagen, however, have revealed new insights into the workings of the brain in Parkinson’s sufferers in a recent study. Professor Shohreh Issazadeh-Navikas is at the helm of this ground-breaking discovery.
“For the first time, we can demonstrate that mitochondria, the vital energy producers within brain cells, particularly neurons, are damaged, resulting in mitochondrial DNA disruptions[LP1]. Our findings establish that the spread of the damaged genetic material, the mitochondrial DNA, causes the symptoms reminiscent of Parkinson’s disease and its progression to dementia,” says Shohreh Issazadeh-Navikas, adding, “Our findings establish that the spread of the damaged genetic material, the mitochondrial DNA, causes the symptoms reminiscent of Parkinson’s disease and its progression to dementia.”
Parkinson’s disease is a chronic neurological ailment that causes symptoms such as difficulties walking, tremors, cognitive issues, and, finally, dementia. Over 10 million people worldwide are affected by the condition. While there is no cure at the moment, several medical therapies can provide respite from its symptoms.
Our findings establish that the spread of the damaged genetic material, the mitochondrial DNA, causes symptoms reminiscent of Parkinson’s disease and its progression to dementia. Our findings establish that the spread of the damaged genetic material, the mitochondrial DNA, causes symptoms reminiscent of Parkinson’s disease and its progression to dementia.
Shohreh Issazadeh-Navikas
Small fragments of mitochondrial DNA spreads the disease
Researchers determined that damage to mitochondria in brain cells develops and spreads when these cells have deficiencies in anti-viral response genes by investigating both human and animal brains. They wanted to know why this damage happened and how it contributed to the sickness.
Their investigation resulted in a startling discovery.
“The mitochondria release little bits of – really DNA – into the cell. When these damaged DNA fragments are misplaced, they become toxic to the cell, forcing nerve cells to eject this toxic mitochondrial DNA,” explains Shohreh Issazadeh-Navikas.
“Given the interconnected nature of brain cells, these toxic DNA fragments spread to neighboring and distant cells, similar to an uncontrolled forest fire sparked by a casual bonfire” she goes on to say.
The dream is a blood sample
Shohreh Issazadeh-Navikas hopes that this study will be the first step toward a better knowledge of the condition and the development of future medicines, diagnostics, and therapy efficacy measurement for Parkinson’s disease. She also stated that “detecting the damaged mitochondrial DNA could serve as an early biomarker for disease development.”
Biomarkers are objective indicators of specific medical disorders that people have. While certain indicators are widespread, such as blood pressure, body temperature, and body mass index, others provide information about specific conditions, such as gene alterations in cancer or blood sugar levels in diabetes. Finding a biomarker for Parkinson’s disease holds a lot of promise for improving future treatments.
“It could be possible that the damage of the mitochondrial DNA in the brain cells leaks from the brain into the blood. That would make it possible to take a small sample of a patient’s blood as a way of diagnosing early on or to establish the favorable response to future treatments.”
Professor Issazadeh-Navikas also sees the prospect of detecting damaged mitochondrial DNA in the bloodstream, which would allow for the condition to be diagnosed or treatment responses to be assessed using a simple blood test.
The next step for the researchers is to look into how mitochondrial DNA damage might be used to forecast disease stages and development. “Furthermore, we are dedicated to exploring potential therapeutic strategies aimed at restoring normal mitochondrial function to rectify the mitochondrial dysfunctions implicated in the disease.”