While research into the relationship between nerve cell insulation and Alzheimer’s disease is ongoing, it is not correct to say that poorly insulated nerve cells directly promote the development of Alzheimer’s disease in the elderly. Alzheimer’s disease is a complex neurodegenerative disorder with numerous contributing factors, and the precise cause is still unknown. Defective myelin actively promotes disease-related changes in Alzheimer’s disease, according to research.
Alzheimer’s disease, an irreversible form of dementia, is the most common neurodegenerative disease in the world. Age is the most important risk factor for Alzheimer’s disease, but why is unclear. Myelin, the insulating layer that surrounds nerve cells in the brain, is known to degenerate with age. The Max Planck Institute (MPI) for Multidisciplinary Sciences in Göttingen has now demonstrated that such defective myelin actively promotes disease-related changes in Alzheimer’s disease. Slowing down age-related myelin damage could lead to new ways to prevent or delay the progression of the disease in the future.
What was I going to do? What happened to the keys? When was that meeting again? It begins with minor memory lapses, then progresses to increasing difficulties orienting, following conversations, communicating, or performing simple tasks. Patients in the final stage are frequently care-dependent. Alzheimer’s disease is a progressive disease that primarily affects the elderly. After the age of 65, the risk of developing Alzheimer’s doubles every five years.
The underlying mechanisms that explain the correlation between age and Alzheimer’s disease have not yet been elucidated. Normal brain function requires intact myelin. “We have demonstrated that age-related changes in myelin promote pathological changes in Alzheimer’s disease.
Klaus-Armin Nave
Signs of aging in the brain
“The underlying mechanisms that explain the correlation between age and Alzheimer’s disease have not yet been elucidated,” says Klaus-Armin Nave, director of the MPI for Multidisciplinary Sciences. He studies the function of myelin, the lipid-rich insulating layer of the brain’s nerve cell fibers, with his team from the Department of Neurogenetics. Myelin ensures that nerve cells communicate quickly and that their metabolism is supported. “Normal brain function requires intact myelin. “We have demonstrated that age-related changes in myelin promote pathological changes in Alzheimer’s disease,” Nave adds.
The researchers investigated the possible role of age-related myelin degradation in the development of Alzheimer’s disease in a new study published in the scientific journal Nature. Their research focused on a common feature of the disease: “Alzheimer’s is characterized by the deposition of certain proteins in the brain,” says Constanze Depp, one of the study’s two first authors. “Amyloid plaques are formed when A peptides clump together.” These plaques form in Alzheimer’s patients many years, if not decades, before the first symptoms appear.” Nerve cells eventually die irreversibly as the disease progresses, and information transmission in the brain is disrupted.
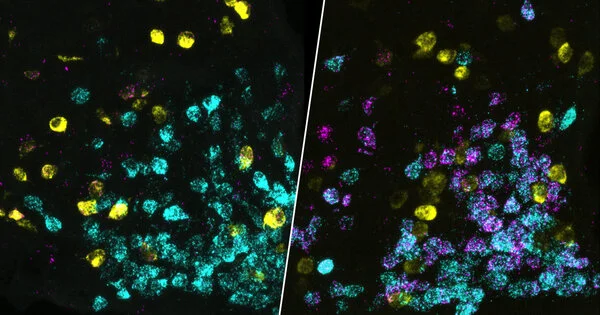
The scientists examined and compared different mouse models of Alzheimer’s disease in which amyloid plaques occur in a similar manner to those found in Alzheimer’s patients using imaging and biochemical methods. They studied Alzheimer’s mice with myelin defects for the first time, which also occur in the human brain at an advanced age.
Ting Sun, the study’s second first author, describes the findings: “We discovered that myelin degradation accelerates the deposition of amyloid plaques in the mice’s brains.” The damaged myelin strains nerve fibers, causing them to swell and produce more A peptides.”
Overwhelmed immune cells
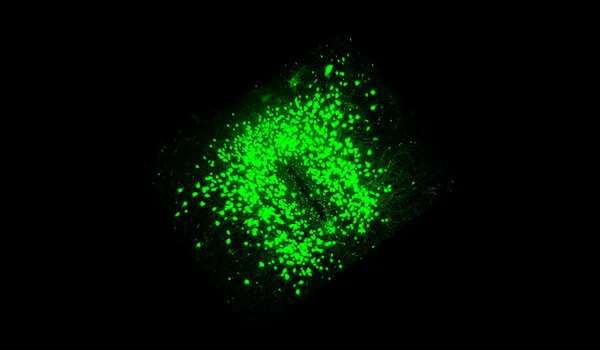
At the same time, myelin defects attract the attention of microglia, which are immune cells in the brain. “These cells are extremely vigilant and constantly monitor the brain for any signs of impairment.” They have the ability to pick up and destroy substances like dead cells or cellular components,” Depp adds. Microglia normally detect and eliminate amyloid plaques, preventing the buildup. When confronted with both defective myelin and amyloid plaques, however, microglia primarily remove the myelin remnants while the plaques continue to accumulate. The researchers believe that the myelin damage has ‘distracted’ or overwhelmed the microglia, preventing them from responding properly to plaques.
A cornerstone for therapeutic approaches
For the first time, the study’s findings show that defective myelin in the aging brain increases the risk of A peptide deposition. “We hope that this will lead to new treatments.” “If we can slow down age-related myelin damage, we may be able to prevent or slow down Alzheimer’s disease,” Nave says.