A study led by the University of Barcelona provides fresh insights into how the molecular pathways involved in regenerative medicine work. The study focuses on tumor necrosis factor-α (TNF-α) and its receptors TNFR, which are significant molecules in biomedicine due to their participation in many disorders such as obesity, type 2 diabetes, inflammatory bowel disease, and cancer.
Professor Florenci Serras of the Faculty of Biology and Institute of Biomedicine at the University of Barcelona (IBUB) leads the work, which is featured in The EMBO Journal’s News & Views section. The project also includes scientists from the University of Buffalo’s Biodiversity Research Institute (IRBIO), the Centre for Genomic Regulation (CRG), and the August Pi i Sunyer Biomedical Research Institute (IDIBAPS).
“In particular, the secreted tumour necrosis factor can recognize and bind to its receptor TNFR, which is located on the membrane of neighbouring cells. As a result of the binding, the TNFR receptor is activated and regulates processes as diverse as cell proliferation, cell death and adaptive immunity,” explains Florenci Serras, a member of the UB’s Department of Genetics, Microbiology and Statistics.
The findings indicate that tumour necrosis factor-α (TNF-α) – a cellular activity modulating protein – has two TNFR receptors that can display completely opposite functions in response to biological tissue injury: specifically, one receptor enhances cell survival and regeneration, while the other can promote cell death.
The study, carried out using the Drosophila melanogaster study model, could contribute to the design of TNFR receptor agonist and antagonist molecules that stimulate the regeneration of epithelial tissues in patients with severe burns, or affected by inflammatory bowel diseases and some cancers.
In particular, the secreted tumour necrosis factor can recognize and bind to its receptor TNFR, which is located on the membrane of neighbouring cells. As a result of the binding, the TNFR receptor is activated and regulates processes as diverse as cell proliferation, cell death and adaptive immunity.
Florenci Serras
Drosophila: a model for studying human diseases
Communication between cells is a decisive process in the development and physiology of organisms. One of the pathways of cell communication is the secretion of molecules — e.g. tumour necrosis factor (TNF-α) — that have specific functions in biological cells, tissues and organs.
“In particular, the secreted tumour necrosis factor can recognize and bind to its receptor TNFR, which is located on the membrane of neighbouring cells. As a result of the binding, the TNFR receptor is activated and regulates processes as diverse as cell proliferation, cell death and adaptive immunity,” explains Florenci Serras, a member of the UB’s Department of Genetics, Microbiology and Statistics.
In the mammalian genome, there are nineteen TNF molecules and twenty-nine TNFR receptors, which reveals the great complexity of their study in the case of the human species. However, some organisms such as the D. melanogaster fly have only one tumour necrosis factor (called Eiger, Egr) and only two TNFRs, which are the Grindelwald (Grnd) and Wengen (Wgn) receptors.
“Thanks to this simplicity, and adding the multiple genetic tools of Drosophila, we have been able to use this model organism to study the regulation and function of TNF-α/TNFR,” says the researcher.
Receptors with opposing functions
Although TNF-α and TNFR receptors are linked to acute and chronic diseases, “it is still not well understood how these components regulate such opposing cellular processes as cell death or cell survival, and even cell proliferation,” Serras stresses.
This study, which will be included in the doctoral thesis to be defended by PhD student José Esteban-Collado, provides evidence that supports the different and opposing functions of TNFR Grnd and Wgn. “On the one hand, the Grnd receptor promotes cell death (apoptosis) to eliminate damaged cells through a TRAF2-dTAK1-JNK signalling pathway in a TNF-α Egr-dependent manner,” says Serras. “In contrast, the Wgn receptor promotes cell survival and regeneration to keep tissues healthy and in good condition, via the TRAF1-Ask1-p38 signalling pathway and without the need for TNF-α Egr,” he adds.
“That is, the first receptor needs the ligand to bind to the receptor, while the second can be activated without interacting with the ligand. Therefore, each TNFR promotes its signalling to achieve different functions,” explains Florenci Serras. “Thus, the communication mechanisms of TNFRs must generate a balance between the activities of the different TNFRs, the molecular signals they set in motion and their dependence — or not — on the ligand (TNF-α),” he points out.
Damaged cells give off molecular signals in healthy cells
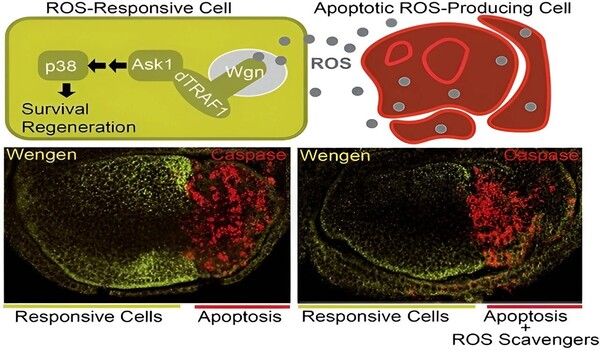
When a cell is dying or damaged, it communicates with healthy cells to replace the non-functional cell with a new one and initiate regeneration of the affected tissue. The research describes how dying cells release reactive oxygen species (ROS), which functional cells in their environment pick up to drive the regeneration process of the affected tissue.
“In a pathological situation or tissue damage, both receptors show different responses. First, the affected tissue produces TNF-α Egr, which binds to Grnd on the membrane. This is internalized and promotes suicide by cell death (apoptosis). At the same time, these cells produce ROS, which spread and reach healthy cells as an alarm signal indicating tissue deterioration,” explains Serras. “The ROS signal activates Wgn in healthy cells directly, without the need for Egr, and consequently triggers the signalling pathway that promotes tissue survival, protection and regeneration,” notes Serras.
The new study’s findings support the hypothesis that ROS from injured tissue can activate Wgn-dependent signaling in healthy surrounding cells, promoting regeneration.
Using an ingenious binary system that permits gene modification in tissue-specific regions, the scientists discovered that TNFR Wgn, but not Grnd, plays a crucial role in p38 kinase activation. “In healthy cells, this p38 will be responsible for setting in motion the entire genetic machinery for tissue repair,” according to Florenci Serras.