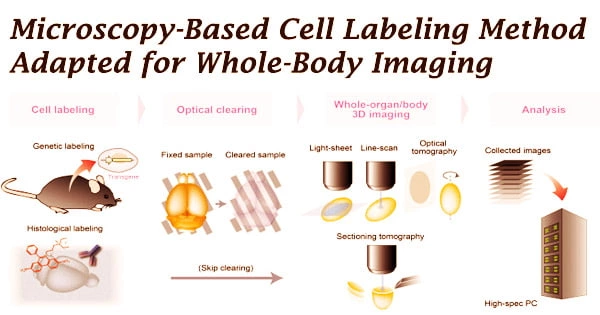
Medical imaging can reveal processes and structures within the body that are typically invisible to the naked eye. Imaging is used by scientists to better understand the intricate activities of cells and organs and to find new approaches to diagnose and cure illnesses. In regular medical practice, pictures of the body assist clinicians in diagnosing illnesses and monitoring the effectiveness of treatments.
Researchers are exploring novel ways for labeling cells or molecules such that they produce signals that may be detected outside the body and translated into relevant pictures in order to portray certain activities in the body. For the first time, a research team from the University of Münster has converted a cell labeling method used in microscopy, known as SNAP-tag technology, for use in whole-body imaging with positron emission tomography (PET).
This technique identifies cells in two phases, and it works for a variety of cell types, including tumor and inflammatory cells. The cells are first genetically engineered to generate a SNAP-tag enzyme on their surface that is specific to the targeted cells. After that, the enzyme is exposed to a suitable SNAP-tag substrate.
The substrate is labeled with a signal emitter and chemically engineered to allow the signal emitter to be delivered to the enzyme after it is recognized and split by the enzyme. The enzyme is changed such that it is no longer active, but the signal emitter remains closely linked to it as a result of the process.
“Through its biological activity, the SNAP-tag enzyme labels itself, so to speak this happens very quickly and without disturbing the natural processes in the organism,” explains Dominic Depke, a biology doctoral student and one of the lead authors of the new study.
Fluorescent dyes are used to identify cells in microscopy, but they are rarely suited for whole-body imaging since their signals are dispersed by larger tissue layers, making them impossible to quantify. The scientists used the radioactive signal emitter fluorine-18 to create a novel SNAP-tag substrate to overcome this difficulty.
The researchers were able to successfully identify tumor cells in animals by injecting this substrate into the circulation and then using PET imaging to see the tumors.
“The exciting thing for us about SNAP-tag technology is that it opens up the prospect of visualizing genetically encoded cells in the body with different imaging modalities and at different temporal stages we call it multiscale imaging,” explains nuclear medicine specialist Prof Michael Schäfers.
“Radioactive signals from fluorine-18 remain stable for only a short time,” adds radiochemist Dr Christian Paul Konken, “but as we can repeat the second labeling step, we can potentially visualize the same cells again and again over days and weeks.”
Microscopy’s high degree of detail allows researchers to investigate how individual cells communicate with one another. Scientists can examine how these cells operate as part of complete organ systems thanks to the big picture view offered by whole-body imaging. As inflammation develops, persists, and disappears, it may become clear what function various cell types play.
“Only by combining all this information can we understand how everything is connected in the body,” says Michael Schäfers.
A small beginning with great potential
“Our investigations are a very first step, in which we have shown that labeling cells with SNAP-tags works, in principle, in living organisms,” emphasizes biochemist Prof Andrea Rentmeister. “What matters here is that the substrate is distributed rapidly in the organism and that it binds exclusively to the cells to be studied.”
The next critical stages will be to see how many cells are required to get a suitably strong signal, as well as if the approach can be used to visualize moving cells within the organism, such as immune system cells.
If the procedure proves effective in the future, it might be useful in future research into immunotherapies, in which the body’s own immune cells are genetically changed in the lab to treat a specific illness. These treatments are currently being used to treat cancer, and they have the potential to help with inflammatory disorders as well. Imaging might aid in the development and refinement of such therapies.
When the scientists presented their findings at a scientific symposium for the first time, they were surprised to learn that colleagues from Tübingen were presenting a similar study at the same time. Both study groups had the same basic idea, a SNAP-tag substrate tagged with fluorine-18, but they were working on it independently. They approached the topic in a different way chemically, but they evaluated the resultant substrates using the same biological model system and came up with comparable results.
“This shows how topical our question is and that our results are reproducible and really promising,” says Michael Schäfers. He adds that the Tübingen team is developing new labeling methods to study immune cells in cancer, while the team in Münster is focusing on inflammatory diseases, so the research complements each other very well. The research team from Münster published their study in the scientific journal “Chemical Communications,” only a few days later the publication from Tübingen was released in “Pharmaceuticals.”
Creating a new substrate for the SNAP-tag
The newly discovered molecule, like other SNAP-tag substrates, is based on benzylguanine, to which the scientists added the radioactive isotope fluorine-18, which is excellent for PET imaging. “Our goal was to design the synthesis in a few quick steps so that we get as strong a signal as possible because fluorine-18 has a short half-life, its radioactivity is reduced by half after every 110 minutes,” explains Christian Paul Konken.
The fluorine-18 did not connect to the proper place on the molecule at first, according to the researchers. “The benzylguanine was apparently too sensitive to be labeled directly with fluorine-18,” says Lukas Rösner, a biochemistry doctoral student, “so we first labelled a small molecule that is insensitive to the necessary chemical reactions the fluoroethylazide and then attached it to the benzylguanine using a click reaction, which is very fast and selective.”
Tests in test tube, cell cultures and the organism
In the first practical experiments in cell cultures, the scientists first tested if the synthesised substrate remained stable while in contact with blood in the test tube, and then looked at how the cells interacted with the substrate. They achieved this by comparing human tumor cells that had the SNAP-tag enzyme genetically integrated to those that did not generate the enzyme.
“We could see very clearly that the radioactivity was only taken up by the cells that produced the SNAP-tag enzyme,” says Dominic Depke. Finally, the team conducted targeted studies on individual mice.
“This step was decisive once again,” explains Michael Schäfers, “because how a molecule behaves in the complex biological environment in a living organism cannot be fully simulated in cell culture or with artificially produced organs.” The researchers were able to demonstrate that once the substrate is injected into the bloodstream, it is rapidly disseminated throughout the body.
In addition, they discovered the routes through which it is expelled. In real creatures, they compared how cancer cells with and without the SNAP-tag enzyme reacted to the substrate. Tumor cells were injected under the skin of mice for this reason, then removed after the inspection to validate the results by autoradiography.