During the COVID-19 pandemic, society learned about the importance of mRNA when scientists and medical experts used it to provide a virus vaccine within a year.
Emmanuel Ho, an associate professor of pharmacy at the University of Waterloo, has developed a unique nanomedicine filled with genetic material known as small interfering RNAs (siRNA) to treat HIV through gene therapy. These siRNAs determine which genes or proteins are switched on or off in our cells, resulting in a 73% reduction in HIV replication.
“This opens the door for new therapeutics in the fight against HIV,” said Dr. Ho, who is one of the Waterloo researchers and entrepreneurs driving health innovation in Canada.
Viruses are smart. They produce Nef proteins to prevent autophagy from occurring. Our process allows our body to fight the viral infection without needing additional drugs.
Dr. Ho
Autophagy, often known as the body’s recycling process, is an important process that eliminates microorganisms such as viruses and bacteria from inside cells. HIV is highly intelligent and creates a protein called Nef, which stops cells from triggering autophagy. This is the first study to produce a combined nanomedicine that can restore autophagy while preventing HIV entry into cells, allowing our body to reactivate its defense mechanism.
Furthermore, HIV contains a gene, CCR5, that permits the virus to enter a cell. To lower HIV infection, siRNAs target both Nef and CCR5. This nanomedicine is designed to be applied vaginally to prevent HIV transmission through sexual contact. As a result, the nanomedicine is designed to be stable without leakage of siRNAs in the acidic vaginal environment but release the siRNA once inside cells.
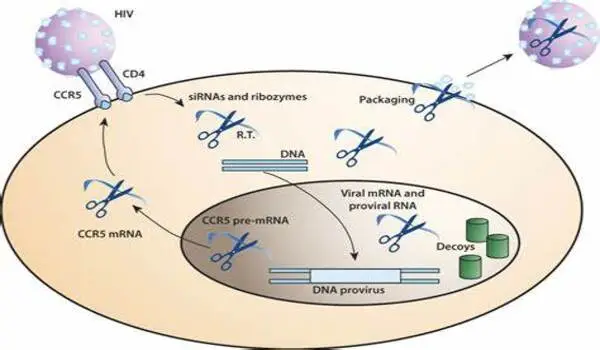
Using RNA in the battle against HIV is a potential strategy that has gained popularity in recent years. RNA-based treatments have the potential to inhibit HIV replication and increase the immune response.
“Viruses are smart. They produce Nef proteins to prevent autophagy from occurring,” Ho explained. “Our process allows our body to fight viral infections without needing additional drugs,” Ho agrees that the next stages include further optimizing the process and improving our understanding of autophagy’s role in virus protection.
“We also hope this will shed some light to develop more alternative approaches to effectively reduce antimicrobial resistance,” Ho added in a statement.