Carbon-based catalysts are catalysts that are based on carbon materials, such as carbon nanotubes, graphene, carbon black, activated carbon, and carbon fibers. Carbon-based catalysts are attractive because of their unique physical, chemical, and electronic properties, as well as their low cost, abundance, and environmental compatibility.
Over the past few decades, carbon-based catalysts have drawn a lot of attention as an affordable substitute for noble metal catalysts in renewable energy systems.
Theoretically, carbon-based catalysts support electrochemical reactions, according to a recent UNIST-affiliated study. The key here is that the combination of structural flexibility, chemical interactions, and the existence of carbon defects allows for an enhancement in catalytic activity even without the use of expensive metal catalysts like platinum (Pt).
The oxygen reduction reaction (ORR) is an important electrochemical reaction and has been widely applied in renewable energy applications, such as hydrogen fuel cells, water splitting, and metal–air batteries (MABs).
Currently, commercial Pt-based catalysts (PBCs) are used extensively for this purpose due to their superior catalytic activity. The research team did highlight that the expensive cost, ease of CO poisoning, and poor durability of Pt prevent the widespread use of PBCs.
Our study is based on numerous theoretical and experimental investigations of carbon materials and is consistent with catalytic experimental results. Moreover, our results can be extended to general chemical reactions in defective carbon materials and will spur research in defect engineering.
The research team
The effectiveness and makeup of catalysts are being gradually enhanced, and carbon-based catalysts are being actively investigated as a leading alternative material. The development of carbon-based catalysts is slowed by the fact that it is unclear why these catalysts promote electrochemical processes.
Carbon-based catalysts have been used in a variety of applications, including:
- Fuel cells: Carbon-based catalysts have been used as a replacement for platinum in fuel cells, which convert the chemical energy of hydrogen or other fuels into electrical energy.
- Chemical synthesis: Carbon-based catalysts have been used in the synthesis of various chemicals, such as pharmaceuticals, agrochemicals, and polymers.
- Environmental remediation: Carbon-based catalysts have been used in the remediation of polluted water and air by oxidizing or reducing organic and inorganic pollutants.
- Energy storage: Carbon-based catalysts have been used in energy storage devices, such as supercapacitors and batteries, due to their high surface area and electrical conductivity.
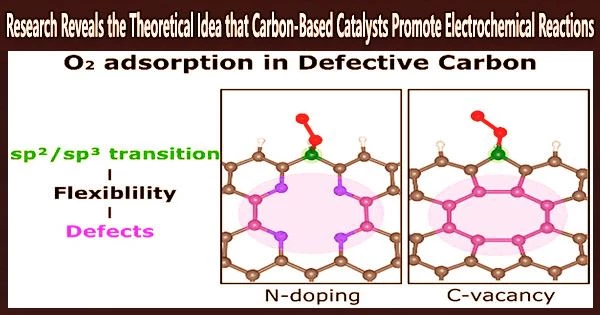
The research team showed in this work how two types of defects, carbon vacancies and pyridinic-N dopants, improve O2 adsorption, a crucial chemical step for activating the ORR.
“Our calculations and structural analysis revealed that defects enable the neighboring edge carbon sites to adsorb O2 by enhancing the structural flexibility and, thus, lowering the energy barrier for the sp2/sp3 transition of the carbon atom,” noted the research team. “Thus, the nonlocal structural environment is as critical as the direct interaction between the adsorption site and adsorbate in the chemical reaction.”
Their findings also demonstrate that pyridinic-N is more structurally stable than carbon vacancies.
“Our study is based on numerous theoretical and experimental investigations of carbon materials and is consistent with catalytic experimental results,” added the research team. “Moreover, our results can be extended to general chemical reactions in defective carbon materials and will spur research in defect engineering.”