The factory Nuvista Pharma Ltd. is situated at the Tongi Industrial area. To implement the laws and protocols that need to be met, the factory is divided into different departments. They are the-
Administration Department
Production Department
Quality Control Department
Packaging Department
Technical Services Department
Store Department
ADMINISTRATION DEPARTMENT
Headed by the Factory Manager the Administration Department is the heart of Nuvista Pharma Limited. Security, welfare activities and the management of staff are only a few of the Administration Department’s responsibilities.
QUALITY CONTROL DEPARTMENT
Quality Operation Department is very important for a pharmaceutical industry to maintain GMP. The concept of total quality management and total quality control refers to the process of striving to produce a perfect product by a series of measures requiring an organized effort by the entire company to prevent or eliminate errors at every stage in production. Although the responsibility for assuring product quality belongs principally to QOD, it involves many departments and disciplines within the company. To be effective it must be supported by a team effort. The QOD is vital for a pharmaceutical industry since it controls and assures for quality of the products starting from the raw materials to the finished product till the customers consume it. All kinds of necessary steps are taken by this department to serve a quality product to the end users.
QUALITY OPERATION
Quality Assurance (QA) Quality Control (QC)
Microbiology
Equipment or instruments used for Quality Operation:
o Hardness Tester, Model: B 24, Western Germany.
o Friability Tester, Model: T A Fed. Rep. Germany.
o Vaccum Oven
o UV
o Dissolution Tester
o Disintegration Tester
o Ultrasonic Bath
o Viscometer
o Karl-Fischer Titrator
o Analytical Balance.
o Centrifuger
o High Performance Liquid Chromatography (HPLC)
QUALITY ASSURANCE
In addition to day to day production also matter day to day. It concerns original design and development product distribution, dispensing upto the administration to the patients Nuvista Pharma Limited gives priority to produce quality product. They believe that only quantity product can satisfy the consumer and a satisfied customer is a great advertiser for the company. For this reason they have taken a stick and inflexible process to check the product for erosion of any kind of defects. For Quality control Nuvista procures Quality raw Materials from their approved source.
Types of work of QA:
1) Raw materials analysis
Source development
Commercial Consignment
Re-testing
Keeping sample & relevant documentation
2) Packaging material analysis
Source development
Commercial Consignment
3) Finish product analysis
In process
Bulk product
4) Water analysis
5) Calibration
6) Validation
7) Packed Product analysis
8) In Process
9) Packet product
10) Stability study
11) Product monitoring
12) Environmental monitoring
13) Dust monitoring
14) Documentation
Flow Chart of Releasing Finished Product:
Collection of sample from the belt as per sampling procedure
Recording of the Quantities of collect sample by analyst on batch packaging record sheet.
Checking of the product
Recording in packed product record sheet
Reporting to the respective officer by analyst (in case of any fault)
Collection sampling advice sheet after packing of a batch
Allocation of the Laboratory Ref. No. to the sampling advice sheet.
Preparing of the report after checking.
Further checking by the officer.
Releasing of the batch to the market by the QC Manager, if satisfactory result comes.
Keeping of the document under supervision of QA after releasing a batch.
Incase of rejection, rejected label (s) will be pasted on the product.
Recording of the rejection details in the rejection register and authorized by QA Manager.
QUALITY CONTROL
Quality control is that part of GMP which is concerned with sampling specification and testing and with organization documentation and release procedures which ensures that the necessary and relevant tests are infact carried out and that materials are not released for sale or supply untill this quality has been judged to be satisfactory.
Activities of the Quality Control Department:
1. Receiving of the samples to be tested from QA department.
2. Issuing release, reject or quarantine advice for each batch of raw material and final product.
3. Assessment of the intermediate products & bulk products for further
processing.
4. Performing all tests procedure for all incoming samples according to the schedule.
5. Maintaining batch wise full quality control tests records & signature of the
Person(s) who perform the test.
6. Performing environmental monitoring tests.
7. Calibrations and standardization of laboratory equipment’s.
8. Ensuring precision and accuracy of all testing methods.
9. Control of all laboratory reagents.
10. Research & development of any new method & its validation.
11. Testing of any return goods.
12. Stability tests for finished products
Working Division of Quality Control Department:
Quality control (QC) department can be further divided into four corresponding sections:
Microbiological section.
Packaging section.
Finished product.
Raw Material.
Lab Facility: Lab facility of Nuvista Pharma Limited is so excellent. Their lab can be described in the following way.
1. Analytical lab
2. Instrumental lab
3. Microbiological lab
Quality control department serves the following functions-
1. Before purchasing of raw materials QC department test the all B.P.& U.S.P specifications of raw materials from the sending sample
2. When raw materials are sent by the source in terms of invoice Q.C. department collects the sample on the basis invoice & labeled an under test sticker.
3. If it meets all E.P., U.S.P., B.P. specification then a passed sticker is labeled.
4. After manufacturing of pharmaceutical preparation Q.C department collects sample from bulk product & test its physical & chemical parameters
5. Q.C department also tests the packaging materials & inspects its printing name corresponding to the product.
6. Q.C department tries to recover tests the quality after final packaging.
7. If any finished product is damaged during transfer to CWH Q.C department try to recover the product as a return goods.
8. After market complain Q.C department tests the product whether the complain is justified or not in respect to the keeping sample
9. Any new products or raw materials supplied by the source is tested by the Q.C respectively for 3 batches’ & set its clear information for monitoring or keeping studies
10. The Q.C department tests water that is used for manufacturing process as it meets its specification.
Quality control department does following tests:
For Raw Materials:
Physical test:
Appearance
Identity
IR
Melting point
HPLC.
Clarity of solution.
Color of solution.
PH (Acidity/Alkalinity).
Specific optical rotation.
Refractive index.
Viscosity.
Bulk density.
Sieve test.
Assay.
Loss on drying or moisture or water content.
Total impurities.
Related substances (HPLC).
Chemical test: potency.
For Bulk product:
A. Solid
Physical test:
1.Dissolution test
2. Disintegration test
3. Uniformity weight
4. Average weight
5. Hardness
6. Thickness
7. Friability
B. Liquid:
Physical test:
1. Appearance
2. Weight per ml
3. Refractive index
4. Viscosity
5. Color
Chemical Test: Active ingredient
Potency
Preservative
C. Dry Granulation Powder:
Physical test:
1. Appearance of dry granules.
2. pH of prepare syrup.
3. Water content.
4. Reconstitution time.
5. Seive test.
6. Inspection of test syrup.
7. Appearances of prepare syrup.
Chemical test: Potency.
D. Semisolid:
Physical test:
1. Apparaence
2. PH
3. Particle size
4. Water content.
Chemical test: Potency.
For packaging Material
The following tests of the packaging material are performed in QC department:
Items Tests
Carton Appearance, weight, moisture content.
Shipping carton Weight, dimension, thickness, appearance
In Process Control (IPC):
IPC test are designed to ensure that the process is under control throughout the manufacturing to have the right things happens at every stage to make a successful batch at the first attempt. The full benefits of IPC come from getting the things’Right the First Time’
. Major advantage of IPC:
• Allows timely action.
• Improves productivity.
• Reduces rejection cost.
• Reduces batch testing at the end.
• Reduces the chance of batch failure.
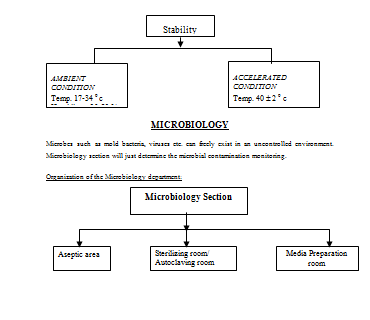
Stability Testing:
The quality control department of Nuvista Pharma Limited also performs stability testing for both the marketed product and the development products.
PRODUCTION DEPARTMENT
Production area is the major section of pharmaceutical industry where men, machine and material are brought together to yield a product of quality. In other words, it is the heart of any pharmaceutical industry. Our training programme would be incomplete without visiting production area.
We saw that the production area of Renata Limited to be well organized and running smoothly. The personnel of this area were very cooperative and well trained.
We would like to express our gratitude to Mr. Md Mizanur Rahman, Production Officer (Solid section) and Mr. Md. Mozammel Haque, Senior Production Officer (Parenteral section). We would like to thank Mr. Sajibul Islam, Mr. Masud, Mr. Tarique, and other officers of this area.
The Production area is meanly classified into two major areas.
1. TABLETTING & CAPSUL DEPARTMENT
2. PARENTERAL DEPARTMENT
TABLETTING & CAPSUL DEPARTMENT
The production unit of Nuvista Pharma Limited is highly maintained to minimize the risk of serious medical hazard due to cross- contamination , dedicated and self contained facilities are available for the production of pharmaceuticals products ,
Objectives
ü Fulfill the market demand
ü High productivity
ü Reproducibility
ü Quality production
Production department has the following units:
- Dispensing
- Granulation
- Compression
- Coating
- Encapsulation
Room available in Production area:
- Change Room
- Office Room
- Dispensing Room
- Liquid Dispensing Room
- Clean equipment Store room
- Washing Bay
- IPC
- Granulation Room
- Compression Room
- Coating Room
To maintain GMP the following facilities are available
ü Adequate space
ü PRW (purified water) supply
ü HVAC (Heating Ventilation Air Conditioning) supply
Total units in solid department:
Both Estrogenic and Non estrogenic production
A. Granulation unit
B. Compression unit
GRANULATION UNIT
Specification of machineries:-
Description | Model No. | Capacity | Manufacturer |
| Tablet Deduster | TD-16 | – | Manesty, UK |
| Tablet Deduster | – | – | Manesty, UK |
| Planetary Mixer (Flame Proof) | PLM-200S | 100 Kg | Gansons, India |
| Rotorgran Oscillating Granulator (Flame Proof) | MK-IV | 30 Kg/hr | Manesty, UK
|
| Drum Hoop Mixer
| – | 200 Lts | Engelsmann, Rhonard
|
| Hako Vacuum Cleaner
| KX-100-15 | 15 Gallons | Hako, USA
|
| Cadmach 35-Station Tabletting Machine
| CMB4-35 | 700020 to 176400 pcs/hr | Cadmach, India
|
| Digital Balance
| BB-244 | 240 gm/ 1mg | Mettler, Switzerland
|
| Manesty B3B 16-Stations Tabletting Machine
| B3B | 21000 to 42000 | Manesty, UK
|
| Manesty BB3B 27-Stations Tabletting Mach.
| BB3B | 45000 to 90000 | Manesty, UK
|
| Capsule Filling Machine
| MF-30 (39/80) | 10000 pcs/hr | PAM, India
|
| Alexanderwerk Granulator
| RAN-70 | 100 Kg/hr | Alexenderwerk, Germany
|
| Glatt Fluid Bed Dryer
| TFO-30 | 30 Kg | Glatt, Germany
|
| Sapphire Fluid Bed Dryer
| 60 Kg | Sapphire, India
| |
| Avery Digital Weighing Balance
| 3359 | 30 Kg/10 gm | Avery
|
| Moisture Determination Balance
| PM-100 | 110 gm/1 mg | Mettler, Switzerland
|
| Drum Hoop Mixer
| 75 Kg | Englesmann
| |
| Digital Platform Scale
| EA150FEG-1 | 75/150 Kg | Sartorius, Germany
|
GRANULATION PROCESS
Steps of granulation process:-
Production area:
- Dispensing room
- Granulation room
- Compression room
- Encapsulation room
- Coating room.
- In-process control (IPC)
- Washing room
Dispensing
Process of dispensing:
- Requisition is given to warehouse for raw materials prior to granulation.
- Raw materials are collected from warehouse through air lock and transferred to a clean dispensing room.
- Before transferring the raw materials to the dispensing room following things should be checked:
ü Materials are checked according to requisition
ü Label check i.e. material is either quarantine, released or rejected
Yellow label indicate—> Quarantine
Green label indicate—> Released
Red label indicate—> Rejected
ü Mfg. date
ü Exp. date.
- Dispensing condition (Temp, RH %) should be maintained.
- Weighing raw materials according to DOS (Dispensing Order Sheet)
- Checking of quantity dispensed.
- Excess material returned to warehouse.
Granulation
Most powders cannot be compressed directly into tablet because-
- They lack the proper characteristics of binding or bonding together into a compact entity and
- They do not ordinarily possess the lubricating and disintegrating properties required for tableting.
For these reasons, drugs must first be pretreated either alone or in combination with filler to form granules that lend themselves to tableting. This process is known as granulation.
Granulation is any process of size enlargement whereby small particles are gathered together into larger, permanent aggregates to render then into free flowing state similar to that of dry sand.
On the other hand, granulation is in part of the pharmaceutical process that attempts to improve the flow of powdered materials by forming sphere like or regularly shaped aggregates called granules.
Reasons of Granulation:
- To prevent segregation of the constituents in the powder mix
- To improve flow properties of the mix
- To improve compression characteristics of the mix
- The granulation of toxic materials will reduce dust generation
- Slightly hygroscopic materials will not form compacts
- Granules are denser and thus occupy less volume per unit weight.
Advantage of Wet Granulation:
- Densification
- Improved compression characteristics
- Reduction in dusting
- Prevention of segregation of powder mix
Disadvantage of Wet Granulation:
- Stability may be a concern for moisture sensitive or thermo labile drugs
- Time, space and equipment required are costly.
Compression
Introduction
Compression is the process of applying pressure to a material. In pharmaceutical tableting an appropriate volume of granules in a die cavity is compressed between an upper and lower punch to consolidate the material into a single solid matrix, which is subsequently ejected from the die cavity as an intact tablet.
The overall tableting cycle is as follows:
- Hopper: Contains the granulated material which is to be tablatted
- Feed frame: Fed by the hopper to hold the granule as it is fed in to the die.
- Feed paddles: Ensures that the granule is keep agitated to correct filling of the die bore.
- Draw-down Cam: The lower punch guide during the first part of the fill stage, adjustable to ensure that the die bore is over filled initially to allow accurate adjustment at weight control.
- Weight control: The lower punch guide during the latter part of the fill stage, adjustable to ensure that as the punch rises, the correct quantity of granule remains within the die, and therefore the tablet dosage is correct.
- Fill Station: The point where the die has been correctly filled.
- Pre-compression rollers: This smaller roller gives the granule an initial pinch to remove as much air as possible before full compression takes place.
- Main compression rollers: Applies full pre-determined pressure to the punches for the final formation of the tablet.
- Compression Station: Where full compression of the tablet is achieved.
- Ejection cam: This forms the lower punch guide during the ejection stage and is adjustable to ensure smooth ejection without damaging tablets.
- Take-off blade: Fitted in front of the feed frame this deflects the tablet down the take-off chute.
- Ejection station: This is the station where the tablet leaves the die for take-off.
- Take-off chute: the chute down which the tablet passes for collection and packaging
Problems during Processing and Compression:-
Tablet production may reasonably exhibit a number of defects during the process of developing formulation and in the routine manufacture of tablets, which are not desirable. These defects arise due to the following reasons:
- Wrong formulation
- Improper setting of tablet machine
- Processing error
Ø Defects related to Tableting Process-
i) Capping It is due air-entrapment in the granular material.
ii) Lamination: It is due air-entrapment in the granular material.
iii) Cracking: It is due to rapid expansion of tablets when deep concave punches are used.
Ø Defects related to Excipient-
iv) Chipping: It is due to very dry granules
v) Sticking
vi) Picking
vii) Binding
Capping:
Capping is the partial or complete separation of top or bottom layer of the tablets from the main body of the tablet.
Cause:
A. Problem of formulation
B. Problem of punch
C. Problem of die
D. Incorrect setting of tablet machine
Chipping:
Chipping is the breaking of the edge of tablet during compression.
- Problem of formulation:
a) Excess moisture:
A certain percentage of moisture is often essential for good compaction. But excess moisture causes softening of granules and causes shipping problem.
Remedy:
– Proper drying
– Then addition of lubricant
b) Lack of lubricant:
Due to the lack of lubricants to the granules, the edge of granules may be broken down during compression.
Remedy:
Addition of sufficient amount of lubricant.
- Problem of punch:
Chipping problem may arise due to the rough surface of the upper punch.
Remedy:
-By changing upper punch
-The surface of the punch is polished by using lubricant.
- Problem of die:
Serious sticking of the granules with the die wall during ejection causes chipping.
Remedy:
1 Die wall is surrounded by tungsten carbide.
2 Inverting the die wall.
- Incorrect setting of tablet machine:
1 Improper setting of feed frame.
2 Incorrect adjustment of sweep off blade.
3 Improper setting of ejection knob.
Remedy:
1 Proper setting of feed frame.
2 Proper adjustment of sweep off blade.
3. Proper setting of ejection knob.
Lamination:
Lamination is the separation of a tablet into two or more distinct layers.
Reason: Air–entrapment during compression and subsequent release on ejection.
The condition is exaggerated by higher speed of turret.
Picking:
‘Picking’ is the term used when a small amount of material from a tablet is sticking to and being removed off from the tablet-surface by a punch face.The problem is more prevalent on the upper punch faces than on the lower ones. The problem worsens, if tablets are repeatedly manufactured in this station of tooling because of the more and more material getting added to the already stuck material on the punch face.
Reason: Picking is of particular concern when punch tips have engraving or embossing letters, as well as the granular material is improperly dried.
Sticking/ Filming:
‘Sticking’ refers to the tablet material adhering to the die wall.Filming is a slow form of sticking and is largely due to excess moisture in the granulation.
Reason: Improperly dried or improperly lubricated granules.
Cracking:
Small, fine cracks observed on the upper and lower central surface of tablets, or very rarely on the sidewall are referred to as ‘Cracks’.
Reason: It is observed as a result of rapid expansion of tablets, especially when deep concave punches are used.
Mottling:
‘Mottling’ is the term used to describe an unequal distribution of colour on a tablet, with light or dark spots standing out in an otherwise uniform surface.
Reason: One cause of mottling may be a coloured drug, whose colour differs from the colour of excipients used for granulation of a tablet.
Double impression:
Double Impression’ involves only those punches, which have a monogram or other engraving on them. If the letters on the tablet are impressed twice, then they are known as double impression.
Reason: At the moment of compression, the tablet receives the imprint of the punch. Now, on some machines, the lower punch freely drops and travels uncontrolled for a short distance before riding up the ejection cam to push the tablet out of the die, now during this free travel, the punch rotates and at this point, the punch may make a new impression on the bottom of the tablet, resulting in ‘Double Impression’.
If the upper punch is uncontrolled, it can rotate during the short travel to the final compression stage and create a double impression.
Binding:
‘Binding’ in the die, is the term used when the tablets adhere, seize or tear in the die. A film is formed in the die and ejection of tablet is hindered. With excessive binding, the tablet sides are cracked and it may crumble apart.
Reason: Binding is usually due to excessive amount of moisture in granules, lack of lubrication and/or use of worn dies.
Weight variation:
a. Unequal size distribution of granules.
b. Poor flow property of granules.
c. Poor mixing
Hardness:
The applied force, which is required for crushing the tablets is called hardness. Hardness depends on-
a. The weight of material
b. The space between the upper and lower punches at the moment of compression.
Cause: Amount of binder.
Remedy:
Adjusting the amount of binder.
Classification of coating
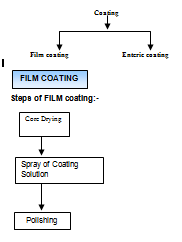
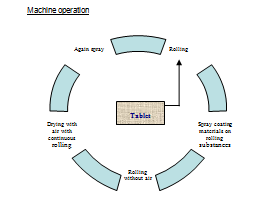
Coating Problem |
Logo bridging
Possible causes
- Inadequate adhesion of the film coating surface characteristics of the products being coated (e.g. hydrophobic substrate).
- Inappropriate design of logo (e.g. too detailed or fine)
- Insufficient plasticizer in film/ high internal stress.
Solutions
- Select opadry II formulation for improved adhesion characteristics
- Modify core formulation to include more hydrophilic ingredients (where possible / or increase core porosity)
v Logo in filling
Possible causes
- Inappropriate design of logo (e.g. to detailed or fine)
- In filling of logo with spray dried coating material
- Logo disappearance can be due to erosion of tablet surface around logo.
Solutions
- See solutions for logo bridging
- Reduce erosion potential by either reformulating core. Changing logo design or modifying curvature of faces of tablet.
- Reduce spray drying potential by increasing spray rate.
Picking / sticking
Possible causes
- Spray rate too high
- Inadequate drying condition
- Pan speed too low
Solutions
- Reduce spray rate
- Improve drying conditions
- Increase pan speed
- Increase atomization air pressure / volume
Twinning
Possible causes
- Spray rate too high
- Pan speed too low
- Inappropriate tablet shape
- Tacky coating formulation
- Spray guns too close to tablet bed.
Solutions
- Reduce spray rate and / or increase atomizing efficiency
- Increase pan speed
Core erosion
Possible causes
- Inherent softness or high friability of core
- Excessive pan speed in coating process
- Spray rate too low
- Low solids content of spray solution
Solutions
Improve mechanical strength of core by increasing compaction force, modifying core formulation or changing process by which core is produced (e.g. use granulation process instead of direct compression)
Peeling
Possible causes
- Low mechnical strength of coating
- Poor adhesion of coating to tablet surface
Solutions
- Select Opadry with improved mechanical strength
- Select Opadry with improved adhesion characteristics
Orange peel /roughness
Possible causes
- Viscosity of coating liquid too high
- Poor atomization of coating liquid
- Drying condition excessive
- Overwetting (causing coating to rub)
Solutions
- Reduce solid contents of coating liquid
- Increasing atomizing air pressure /volume.
CAPSULE
Capsule is a solid dosage form, in which medicaments are enclosed in a capsule shell, made from gelatin. Generally we use empty hard gelatin shell. Capsules shells are made of two parts-body and cap. Medicaments in the form of powders or granules, are filled into the body and locked with cap-the form of dosage form is called capsule.

Problem during capsule manufacturing:
Usually the following problem occurs during encapsulation:
- Capsule shell size variation
- Capsule shell color variation
- Shell lock
- Pore of shell
- Shell cutting is not correct
- Two shell attaches to each other
- Pellets become clump together
- Charge develops on pellets surface on frequent agitation and they stick to each other hampering the flow rate. By using talc, mg stearate the following problem can be resolve.
- Weight variation
Equipment used in Sterile Manufacturing:-
- Capsule Filling machine ………. P+am (India)
PARENTERAL DEPARTMENT
Injections are sterile and pyrogen free products that intended to be administrated in the body with the help of syringe or needles through various route such as IV, IM etc. As the products directly go to the circulation, they must be free from any microbial contamination, toxic compound that should process & exceptionally high level of purity.
Nuvista Pharma has separate section for injections which consists several subunits-
- Aseptic room for filling and sealing
- Sterilization room (autoclaving and terminal sterilization)
Facility status for aseptic area:
- Room temperature: 20ºC ± 2ºC.
- Room humidity: 45% to 60%
- Air velocity of laminar flow: Horizontal 84+20 ft/min, Vertical 56+10 ft/min.
- Air change not less than 20 per hour.
Environmental cleanliness standard:
It is defined in term of maximum number air borne particle rather than a define particle size in a given volume of air –
Particle | British standard | U.S. Federal Standard
| ||||
Class | No./ft3 | No./m3 | Class | No./ft3 | No./m3
| |
0.5 μ | 1 | 86 | 3000 | 100 | 100 | 3500 |
5 μ | 1 | 0 | 0 | 100 | 0 | 0 |
0.5 μ | 2 | 8495 | 3,00,000 | 1000 | 1000 | 3,50,000 |
5 μ | 2 | 57 | 2000 | 1000 | 65 | 23,000 |
Cleaning and sanitation for aseptic area:
- Fumigation of the room: this is done with 2.5% savlon in DM water.
- UV irradiation for whole night.
Sterilize products and equipments:
| Type | Condition | Materials
|
| Moist heat sterilization
| Autoclaving 121ºC at 15 Kg/cm2 for 30 minutes.
| Finished products (Ampoule, vial), Rubber closure, container, membrane filter, SS vat, filling machine.
|
| Dry heat sterilization
| Heating at 220 ºC for 2 hours. (Double door)
| Equipment, empty ampoules, vials, bottles etc.
|
Water treatment plant:-
In NUVISTA PHARMA water used in liquid division mean purified water, drinking water, normal water, distilled water, WFI (water for injection) all are supplied by sterile division.
Water filtrated by 0.2μ filter. In some cases specially water wastage in washing section in sterile division are reused by filtration and pass through IR. Drinking water also supply in the head office through container.
Fig: Water Treatment Plant Flow Chart of Nuvista pharma Ltd.
Equipment used in Sterile Manufacturin
ROTA Filling & Sealing Machine for Ampoules, England
Filtration Machine – Palltronic, Germany
Manufacturing Vessel (50 Liter)
Laminar Air Flow Unit, Franz Ziel, Germany.
Hot Air Sterilizer
Dissolved Oxygen Analyzer
Distilled Water Plant
Linden Autoclave (17) Steam Generator
Sterile section has terminal products and aseptic product producing zone
Terminal products:
The products which are not heat sensitive are called terminal products. The products are autoclaved after filing and sealing. Mainly injections and water for injections are terminal products
Aseptic products:
The products that are heat sensitive are aseptic products. Autoclaved is done by filtration process. Double filter paper is used. The pore size of first filter paper is 0.5 mµ. The pore size of second filter paper is 0.2 mµ. no bacteria can pass through the pores of 0.2 mµ. Mainly eye drops and insulin are considered as aseptic products
Packaging department
Packaging is the process by which the pharmaceutical products are suitable paked, in such way that they should retain their therapeutic effectiveness from the time of their packaging to consume by the consumers & it also helps to withstand the stress during transport.
Nuvista Pharma Limited has a well-established packaging department.
Packagings are of two types:
- Primary packaging (Direct contact with the product eg.alluminium foil)
- Secondary packaging (No contact with the product eg. cartoon case etc)
Primary packaging has two categories:
- Blister packaging
- Strip packaging.
In Nuvista Pharma Blistering machine are:
- HOONG A- Korea
- HOONG B- Korea
- HOONG C- Korea
- FORMPACK ( Injection )
- GANSON-India ( Tablet strip)
- Batch Printing Machine:
- Morico, Japan
Two forms of blistering:
- Alu – alu
- Alu – PVC
Material Used in Packing Section:
- Alu.Foil(Blister/Strip)
- PVC/PVDC Film
- Alu-Alu Foil (Bottom).
- Bottle/Alu.Tube.
- PP Cap.
- Label.
- Unit Carton.
- Insert.
- Plastic Spoon.
- Stopper.
- Shipping Carton.
Ø Plastic Cap.
Different parts of Blistering machine:
- Heating device
- Forming station
- Filling station
- Sealing station
- Cooling station
- Draw off
- Production tray
- Slit heater
- Code embossing station
- Cutting station
- Coiler
- Conveyor belt
Flow chart of how a Blister machine works during PVC-Aluminium Blister:
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
Pre heater (110 °C) | Forming plate (Air pressure used) | Hand loading of tab/cap. | Sealing (190 °C) | Cooler (to ensure the product is not exposed to heat) | Batch no. | Cooling | Slitting | Draw | Cut |
Important for primary packaging:
- Plug area create pocket by plugging (Alu-Alu), In case of Alu-PVC forming create pocket with the help of air pressure & temp (130º-160 ºC).
- Tablets are manually arranged into production trey.
- Incase of automatic filling, hopper chute channel has two parts
> Chute vibrator
> Feeding roller.
- Top holders contain Aluminium foil.
- Sealing temperature 150 ºC to 210 ºC.
- Scoring is done by slitter. In case of alu-alu, no heat is required. Whereas in alu-PVC 80 ºC to 100 ºC temperature is required.
- Waste coiler coil the wastage remaining after cutting.
- Drawer draws the blistering materials to the cutting area.
The test that are performed by this section for packaging materials are-
| Materials Name | Test specification |
| PVC | Color, width, thickness, weight per unit area etc.
|
| Cotton | Appearance, weight, moisture.
|
| Shipping carton | Weight, diameter, thickness.
|
| Inner carton | Height and level description
|
| Plastic cap | Appearance, weight, length, volume etc
|
| Dropper | Appearance, length, weight and plastic cover.
|
| Tape (adhesive) | Appearance, width, Adhesiveness.
|
| Bottles, Ampoules | Height, volume, capacity, diameter, machine acceptance etc.
|
Test for Primary packaging:
- Leak test
- Primary quality
- Batch No. and Exp. Date
- Perforation
- Sealing
- Clarity of Pocket
Leak Test:
Leak test is done by the leak test machine after completing blistering and stripping.
Blister / strip are kept under the pored vehicle in the container which contains purified water. Air pressure is given by switching on. Maximum 760 mmHg pressure can be given. But usually 400mmHg is given in case of big pocket and 600mmHg is given in small pocket.
Test for secondary Packaging:
- Box, Leaflet, Strip
- Batch No., Mfg. Date, Price
- Color & printing of the Box
When the primary packaging is completed then the tablet or capsule containing blister or strips are checked. And finally the strips or blisters are made ready for secondary packaging.
A fixed number of tablets or capsules are packed into each strip or blister. And a fixed number of strips or blisters are packed into each packet. The packets are then poured into the box.
QUALITY CONTROL DEPARTMENT
Quality Operation Department is very important for a pharmaceutical industry to maintain GMP. The concept of total quality management and total quality control refers to the process of striving to produce a perfect product by a series of measures requiring an organized effort by the entire company to prevent or eliminate errors at every stage in production.
Although the responsibility for assuring product quality belongs principally to QOD, it involves many departments and disciplines within the company. To be effective it must be supported by a team effort.
The QOD is vital for a pharmaceutical industry since it controls and assures for quality of the products starting from the raw materials to the finished product till the customers consume it. All kinds of necessary steps are taken by this department to serve a quality product to the end users.
Quality Operation
Equipment or instruments used for Quality Operation:
- Hardness Tester, Model: B 24, Western Germany.
- Friability Tester, Model: T A Fed. Rep. Germany.
- Vaccum Oven
- UV
- Dissolution Tester
- Disintegration Tester
- Ultrasonic Bath
- Viscometer
- Karl-Fischer Titrator
- Analytical Balance.
- Centrifuger
- High Performance Liquid Chromatography (HPLC
QUALITY ASSURANCE
In addition to day to day production also matter day to day. It concerns original design and development product distribution, dispensing upto the administration to the patients Nuvista Pharma Limited gives priority to produce quality product. They believe that only quantity product can satisfy the consumer and a satisfied customer is a great advertiser for the company. For this reason they have taken a stick and inflexible process to check the product for erosion of any kind of defects. For Quality control Nuvista procures Quality raw Materials from their approved source.
Types of work of QA:
1) Raw materials analysis
- Source development
- Commercial Consignment
- Re-testing
- Keeping sample & relevant documentation
2) Packaging material analysis
- Source development
- Commercial Consignment
3) Finish product analysis
- In process
- Bulk product
4) Water analysis
5) Calibration
6) Validation
7) Packed Product analysis
8) In Process
9) Packet product
10) Stability study
11) Product monitoring
12) Environmental monitoring
13) Dust monitoring
14) Documentation
Flow Chart of Releasing Finished Product:
Collection of sample from the belt as per sampling procedure
Recording of the Quantities of collect sample by analyst on batch packaging record sheet.
Checking of the product
Recording in packed product record sheet
Reporting to the respective officer by analyst (in case of any fault)
Collection sampling advice sheet after packing of a batch
Allocation of the Laboratory Ref. No. to the sampling advice sheet.
Preparing of the report after checking.
Further checking by the officer.
Releasing of the batch to the market by the QC Manager, if satisfactory result comes.
Keeping of the document under supervision of QA after releasing a batch.
Incase of rejection, rejected label (s) will be pasted on the product.
Recording of the rejection details in the rejection register and authorized by QA Manager.
Quality Control
Quality control is that part of GMP which is concerned with sampling specification and testing and with organization documentation and release procedures which ensures that the necessary and relevant tests are infact carried out and that materials are not released for sale or supply untill this quality has been judged to be satisfactory.
Activities of the Quality Control Department:
1. Receiving of the samples to be tested from QA department.
2. Issuing release, reject or quarantine advice for each batch of raw material and final product.
3. Assessment of the intermediate products & bulk products for further
processing.
4. Performing all tests procedure for all incoming samples according to the schedule.
5. Maintaining batch wise full quality control tests records & signature of the
Person(s) who perform the test.
6. Performing environmental monitoring tests.
7. Calibrations and standardization of laboratory equipment’s.
8. Ensuring precision and accuracy of all testing methods.
9. Control of all laboratory reagents.
10. Research & development of any new method & its validation.
11. Testing of any return goods.
12. Stability tests for finished products
Working Division of Quality Control Department:
Quality control (QC) department can be further divided into four corresponding sections:
ü Microbiological section.
ü Packaging section.
ü Finished product.
ü Raw Material.
Lab Facility: Lab facility of Nuvista Pharma Limited is so excellent. Their lab can be described in the following way.
1. Analytical lab
2. Instrumental lab
3. Microbiological lab
Quality control department serves the following functions-
1. Before purchasing of raw materials QC department test the all B.P.& U.S.P specifications of raw materials from the sending sample
2. When raw materials are sent by the source in terms of invoice Q.C. department collects the sample on the basis invoice & labeled an under test sticker.
3. If it meets all E.P., U.S.P., B.P. specification then a passed sticker is labeled.
4. After manufacturing of pharmaceutical preparation Q.C department collects sample from bulk product & test its physical & chemical parameters
5. Q.C department also tests the packaging materials & inspects its printing name corresponding to the product.
6. Q.C department tries to recover tests the quality after final packaging.
7. If any finished product is damaged during transfer to CWH Q.C department try to recover the product as a return goods.
8. After market complain Q.C department tests the product whether the complain is justified or not in respect to the keeping sample
9. Any new products or raw materials supplied by the source is tested by the Q.C respectively for 3 batches’ & set its clear information for monitoring or keeping studies
10. The Q.C department tests water that is used for manufacturing process as it meets its specification.
Microbiological assay and other tests:
The department of Microbiology performs the role of immense importance to follow the GMP and to formulate as well as to implement the SOPS.
The overall activity profile of the microbiological section of Q.C department of Nuvista Pharma can be presented briefly in the following way——–
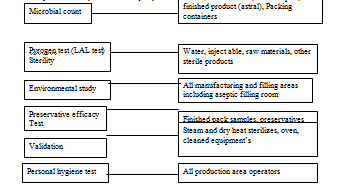
Instruments used in Microbiology Laboratory:
▪ Particle counter
▪ Air sampler (RCS)
▪ Incubator
▪ Anaerobic jar
▪ Centrifuge machine
▪ Water bath
▪ Freeze to preserve bacteria
▪ PH meter
▪ Colony counter
▪ Microscope
▪ Laminar flow machine
▪ Hot oven
▪ Dust collector
▪ Autoclave
▪ Balance
▪ LAL Testing Kit
TECHNICAL SERVICES DEPARTMENT
The engineering department is responsible for regular maintenance of the plant, machines, services and sanitation to help achieve smooth production and safe environment.
The were some function of this department-
• Installation of production equipment.
• Performance of equipment.
• Machine capabilities.
• Maintenance of the building.
• Maintenance of the fire alarming system.
• Validation of laboratory equipment’s.
• Calibration of laboratory equipment’s.
• Maintenance of heating, ventilation and air conditioning system (HVAC).
• Maintenance and supply purified water for treatment.
• Well controlled of drainage system.
• Supply of electricity of its own arrangement.
• Maintenance of effluent water treatment plant.
Nuvista Pharma Limited has a well-equipped engineering department. It is divide into three sections.
Civil
Electrical and
Mechanical
Civil: All Construction works (any new construction and repairing) is done under this section.
Electrical: Daily electric supply to the industry is the main job of this section arises to the supply of electricity, and then this section takes emergency measurements. Mechanical: This section does Installation and validation of production machinery and their machinery’s. The following services are also under the control of mechanical section.
1. Air conditioning
2. Vacuum.
3. Steam supply.
4. Compressed air. 5. Water supply.
Cooling system of Nuvista Pharma Limited:
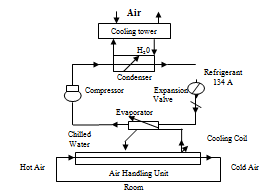
WAREHOUSE
Ware House is the large area where all the raw materials, packaging materials and the finished products are stored. From the Ware House the raw materials are sent to the production unit. Again the finished products are stored in the Ware House.
Warehousing is normally the largest in terms of area special attention is paid which is focused on maintaining cleanliness freedom form infestation and order orderliness.
Different Units of Warehouse:
v Storage for Raw material.
v Storage for Packing material.
v Storage for Finished product.
v Storage for Thermolabile product (Foster/cold storage).
Areas of Warehouse:
1. Quarantine area: After receiving, raw materials and packaging materials are kept here for QA approval. Area is located in the front portion of the building.
2. Released area: Approved raw materials and packaging materials are generally stored in the central place of the warehouse with great safety.
3. Rejected area: Rejected raw materials, packaging materials, finished products are stored here with great care. The rejected area is located at corner portion of the warehouse in a separate room, where entry is strictly regulated.
4. Finished Products area: Finished products are stored here for delivery.
5. Special area: It includes cold room, freeze room for poisonous materials, room for flammable materials.
Activities of Warehouse:
Warehouse activities can be stated as follows: (related to raw and packaging materials)
ü Arrival of materials.
ü Invoice checking.
ü Physical inspection & receipt/discrepancy report.
ü Quarantine storage.
ü QA sampling.
ü Leveling in the container.
ü Entry in SAP.
ü QA release/ rejected materials.
ü Dispensing of released/ rejected materials.
ü Dispensing / Distribution.
Objectives of proper receiving, issuing and storage of goods in Nuvista Pharma –
Measures Target:
- Proper entry of all receipts & issues into the computer / Kardex 100 %
- Timely issue of goods to production department & central warehouse as per requisition. 100 %
- To minimize handling loss of issuing materials
Raw Materials NMT.500 %
Packaging Materials NMT.500 %
Finished Goods (Glass Bottles) NMT.500 %
- Maintain FEFO (1st expelled 1st output) / FIFO (1st input 1st output) 100 %
- Sometime maintain LIFO (last in first out) in case of raw material.
- To follow guidelines as per cGMP / GSP 100 %
Objectives of smooth receiving & supplying of packing materials, Raw materials (Solids, Liquids) in Nuvista Pharma –
Measures Target:
- Timely unloading ½ hour after receiving the documents
- Tallying the quantity with delivery challan against ½ hour after receiving purchase order / L / C & preparing G.R.N. the challan.
- Orderly placement of received materials immediately after at quarantine area of the store receiving the materials
- Shelving passed materials at respective shelves quickly transferring of the packing materials store from quarantine.
- Proper entry of all receipts & issues into the Bincard 100 %
- Timely issue of packing materials to production department as per requisition. 100 %
- To minimize handling loss of issuing materials
Packaging 98 %
Solid materials 99.5 %
Liquids 99.5 %
- Maintain FEFO (1st expelled 1st output) / FIFO (1st input 1st output) 100 %
- To follow guidelines as per cGMP / GSP 95 %
Objectives of smooth receiving & supplying of finished goods in Nuvista Pharma:
Measures Target:
- Tallying the goods mentioned in quickly after receive finished goods transfer note goods transfer note.
- Proper entry of all receipts & issues into Cardex / Bincard 100 %
- Timely delivery of goods as per prescribed vat challan & gate pass of custom authority 100 %
- Preparing daily statement 100 %
- Maintain FEFO (1st expelled 1st output) / FIFO (1st input 1st output) 100 %
- To follow guidelines as per cGMP / GSP 95 %
Some common terms of Ware house:
Sampling
As the process of taking a small portion from a lot for test and analysis to show the quality of the whole lot. The purpose of sampling and subsequent testing is to provide an effective check on the quality of the product or substances being processed.
Sampling quantity
Sampling quantity should be the double of one complete test.
Lot
A batch or number of batches in a consignment.
Batch
A quantity of the product or material which is processed in one run following manufacturing USP. ]
Campaign
A campaign means number of batches manufactured without any interruption or product change.
Handling
The term handling means checking according to invoice/challan and other documents during receiving of the materials.
Preservation
The term preservation means the materials are stored in different conditions according to its nature of stability i.e. to maintain a specific temperature and relative humidity.
Dispensing
Dispensing means the materials are supplied to the production areas by weighing according to the proper document and release it from the RM Store.
Quarantine The term quarantine means the material is not ready for use and it is under test after received. So a quarantine label is attached to the container.
FIFO The term FIFO stands for First In First Out.
Re-test The term re-test means the samples are needed to be repeated analysis for identify vs. previous documentation and it has been done either 3/6/12 months.
COMMENTS
v Comments about Tablet & Capsule Department:-
- Highly sophisticated HVAC system and AHU are used to condition, monitor and supply clean air to the working zone.
- Temperature and moisture levels are maintained at the desired level
- All modern, effective and necessary equipments required for granulation are available.
- Efficient and experienced production officers.
- Well trained manpower.
- Quality is maintained by Quality Compliance unit.
Opportunities:-
- Productivity may be increased by proper utilization of granulation machinery.
- All the hand filling process must be replaced by automatic filling process
v Sterile Department:-
- Highly sophisticated HVAC system and AHU are used to condition, monitor and supply clean air to the working zone.
- Neat and clean working area.
- Particle and organisms are strictly controlled specially in sterile area.
- Clean room class is maintained each and every area of sterile section.
v Quality Assurance Department:
- Quality Assurance Department is really proper oriented.
- Space for the quality control section is really inadequate. It needs to be expanded urgently in order to keep up the quality of the product.
CONCLUSION
Man is the best creation of God. He gained this rank and position by dint of education. Education makes a man a perfect human being. Education aware him of his duty to the family, to the society and there by to the nation. Having had the pharmaceutical education, we now realize that we shall be able to tender our services to man kind to some extent.
The objective of the industrial training was the partial fulfillment of courses and for achievement of practical experience about manufacturing of quality drugs.
We are fortunate enough to be gainer of the opportunity to receive training from a company like ‘Nuvista Pharma limited’. We would like to express our sincere gratitude to ‘Nuvista Pharma limited’ for their constant professional guidance, conceptual teaching and proper training.
‘Nuvista Pharma limited’ has been manufacturing pharmaceutical finished products and market leader of hormonal contraceptives. Quality of Nuvista Pharma products have gained widespread trust and confidence due to
v Commitment to the highest quality products.
v Stringent quality control procedures.
v Highest cGMP standards.
v Raw materials from the best source.
v Highest quality packaging materials.
v World class formulation technology.
v Diversified and sophisticated dosage forms.
In conclusion, I hope that, Nuvista Pharma Ltd. has succeeded to achieve all the objectives of its establishment and continue its success in future by taking all necessary steps which should be required for its growth.





![Thesis Paper on Performance Analysis and Budgetary Control Activities of Trade Vision Limited [Part-1]](https://assignmentpoint.com/wp-content/uploads/2013/04/images-18-110x55.jpg)









