Rice University researchers created an electrochemical reactor that has the potential to significantly reduce energy usage for direct air capture, which is the removal of carbon dioxide straight from the environment.
The novel reactor design could help to solve the pressing problem of emissions’ effects on the climate and biosphere by allowing for more adaptive and scalable carbon dioxide mitigation measures.
A study in Nature Energy describes the specialized reactor as having a modular, three-chambered structure with a carefully engineered porous solid electrolyte layer at its core. Haotian Wang, a Rice chemical and biomolecular engineer whose lab has been researching industrial decarbonization and energy conversion and storage solutions, said the work “represents a big milestone in carbon capture from the atmosphere.”
Our reactor can efficiently split carbonate and bicarbonate solutions, producing alkaline absorbent in one chamber and high-purity carbon dioxide in a separate chamber. Our innovative approach optimizes electrical inputs to efficiently control ion movement and mass transfer, reducing energy barriers.
Zhiwei Fang
“Our research findings present an opportunity to make carbon capture more cost-effective and practically viable across a wide range of industries,” said Wang, the corresponding author on the study and associate professor of chemical and biomolecular engineering.
The device has demonstrated industrially relevant rates of carbon dioxide regeneration from carbon-containing liquids. Its performance criteria, including as long-term stability and adaptation to various cathode and anode reactions, demonstrate its suitability for large-scale industrial applications.
“One of the major draws of this technology is its flexibility,” Wang said, revealing that it works with a variety of chemistries and can be used to cogenerate hydrogen. “Hydrogen coproduction during direct air capture could translate into dramatically lower capital and operation costs for downstream manufacturing of net-zero fuels or chemicals.”
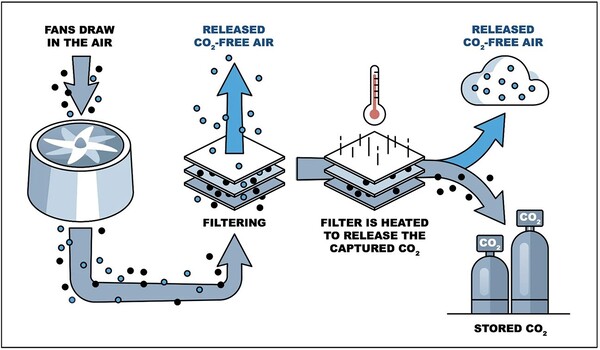
The novel approach provides an alternative to the utilization of high temperatures in direct air capture processes, which frequently entail passing a mixed gas stream through high-pH liquids to filter out carbon dioxide, an acid gas. This first stage in the process connects the carbon and oxygen atoms in the gas molecules to other compounds in the liquid, producing new bonds with variable degrees of strength depending on the chemical used to trap the CO2. The next significant stage in the process is to extract the carbon dioxide from these solutions, which can be accomplished via heat, chemical reactions, or electrochemical techniques.
Zhiwei Fang, a Rice postdoctoral researcher who is a study co-first author, said conventional direct air capture technologies tend to use high-temperature processes to regenerate carbon dioxide from sorbent, or the carbon dioxide-filtering agent.
“Our work focused on using electrical energy instead of thermal energy to regenerate carbon dioxide,” Fang said, adding that the approach has several additional benefits, including it works at room temperature, needs no additional chemicals and generates no unwanted byproducts.
The types of chemicals used to trap the carbon dioxide have different drawbacks and advantages. Amine-based sorbents are the most widely used, in part because they tend to form weaker bonds which means less energy is required to take the carbon dioxide back out of the solution. However, they are highly toxic and unstable. Even though basic water-based solutions using sorbents like sodium hydroxide and potassium hydroxide are a greener alternative, they require much higher temperatures to release the carbon dioxide back out.
“Our reactor can efficiently split carbonate and bicarbonate solutions, producing alkaline absorbent in one chamber and high-purity carbon dioxide in a separate chamber,” Wang told me. “Our innovative approach optimizes electrical inputs to efficiently control ion movement and mass transfer, reducing energy barriers.”
Wang thinks that the research will encourage more industries to seek sustainable practices and accelerate the transition to a net-zero future. He said that this study, as well as others in his lab over the years, illustrate Rice’s strategic focus on sustainable energy innovation.