Nanosensors are typically highly specialized devices that rely on nanoscale materials and fabrication techniques, and their development often involves a range of complex processes and materials.
Engineers at Macquarie University have developed a new technique for producing nanosensors that is much less carbon-intensive, much cheaper, much more efficient, and much more versatile, significantly improving a key process in this trillion-dollar global industry.
Instead of the traditional method of heating materials to high temperatures, the team discovered a way to treat each sensor with a single drop of ethanol. Their study, titled ‘Capillary-driven self-assembled microclusters for highly performing UV detectors,’ was published in the Journal of Advanced Functional Materials.
“Nanosensors are usually made up of billions of nanoparticles deposited onto a small sensor surface – but most of these sensors don’t work when they’re first fabricated,” says corresponding author Associate Professor Noushin Nasiri, head of the Nanotech Laboratory at Macquarie University’s School of Engineering.
Nanosensors are usually made up of billions of nanoparticles deposited onto a small sensor surface – but most of these sensors don’t work when they’re first fabricated.
Professor Noushin Nasiri
The nanoparticles form a network held together by weak natural bonds, which can cause so many gaps between nanoparticles that they fail to transmit electrical signals, causing the sensor to fail.
Associate Professor Nasiri’s team discovered the discovery while working to improve ultraviolet light sensors, the key technology behind Sunwatch, which earned Nasiri a Eureka Prize nomination in 2023.
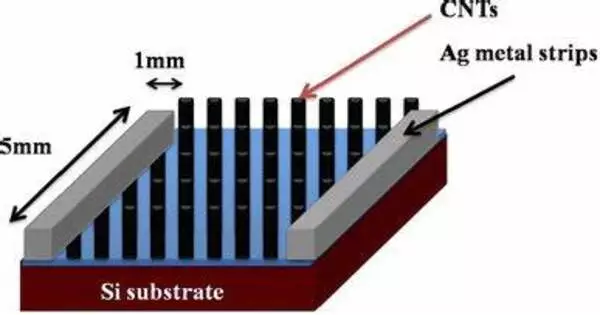
Nanosensors have a high surface-to-volume ratio because they are made up of layers of nanoparticles, making them extremely sensitive to the substance they are designed to detect. However, most nanosensors do not function effectively until they are heated in a time-consuming and energy-intensive 12-hour process that uses high temperatures to fuse layers of nanoparticles, creating channels that allow electrons to pass through layers and allow the sensor to function.
“Most polymer-based sensors are destroyed in the furnace, and nanosensors with tiny electrodes, such as those in a nanoelectronic device, can melt.” “Many materials cannot currently be used to make sensors because they cannot withstand heat,” explains Associate Professor Nasiri. However, the Macquarie team’s new technique avoids this heat-intensive process, allowing nanosensors to be made from a much broader range of materials.
“Adding one droplet of ethanol to the sensing layer without putting it in the oven will help the atoms on the surface of the nanoparticles move around, and the gaps between nanoparticles will disappear as the particles join together,” Associate Professor Nasiri explains. “We demonstrated that ethanol significantly improved the efficiency and responsiveness of our sensors, far exceeding what would be obtained after heating them for 12 hours.”
The new method was discovered after the lead author of the study, postgraduate student Jayden (Xiaohu) Chen, accidentally splashed some ethanol onto a sensor while washing a crucible, an incident that would normally destroy these sensitive devices.
“I thought the sensor was destroyed, but later realised that the sample was outperforming every other sample we’ve ever made,” Chen says.
Associate Professor Nasiri says that the accident might have given them the idea, but the method’s effectiveness depended on painstaking work to identify the exact volume of ethanol used.
“When Jayden discovered this result, we went back and tried different amounts of ethanol very carefully.” “He was testing over and over to see what worked,” she explains. “It was like Goldilocks – three microlitres was too little and didn’t do anything, ten microlitres was too much and wiped out the sensing layer, and five microlitres was just right!”
The team has patents pending for the discovery, which has the potential to make a huge impact in the world of nanosensors.
“We developed a recipe for making nanosensors work, and we tested it with UV light sensors, as well as nanosensors that detect carbon dioxide, methane, hydrogen, and other gases – the effect is the same,” Associate Professor Nasiri says.