Engineers have created a scalable method for optimizing a silicon anode priming method that improves lithium-ion battery performance by 22% to 44%. Silicon anode batteries have the potential to transform energy storage capabilities, which is critical for meeting climate goals and realizing the full potential of electric vehicles. The irreversible depletion of lithium ions in silicon anodes, on the other hand, poses a significant barrier to the development of next-generation lithium-ion batteries.
Rice University’s George R. Brown School of Engineering researchers have developed a scalable method to optimize prelithiation, a process that coats silicon anodes with stabilized lithium metal particles (SLMPs) to help mitigate lithium loss and improve battery life cycles.
Sibani Lisa Biswal’s Rice lab discovered that spray-coating the anodes with a mixture of the particles and a surfactant improves battery life by 22% to 44%. Initially, battery cells with a greater amount of coating had greater stability and cycle life. However, there was a drawback: when the battery was cycled at full capacity, a larger amount of the particle coating resulted in more lithium trapping, causing the battery to fade faster in subsequent cycles.
The research was published in the journal ACS Applied Energy Materials.
The volume of a silicon anode will vary as the battery is cycled, which can break the SEI or otherwise make it unstable. We want this layer to remain stable throughout the battery’s later charge and discharge cycles.
Quan Nguyen
Would replacing graphite in lithium-ion batteries with silicon significantly improve their energy density? how much energy is stored in relation to weight and size? because graphite, a carbon-based material, can hold fewer lithium ions than silicon. Each lithium-ion requires six carbon atoms, whereas one silicon atom can bond with up to four lithium ions.
“Silicon is one of those materials that has the potential to significantly improve the energy density for the anode side of lithium-ion batteries,” Biswal explained. “That’s why there’s currently this push in battery science to replace graphite anodes with silicon ones.”
However, silicon has other properties that present challenges.
“One of the major problems with silicon is that it continually forms what we call a solid-electrolyte interphase or SEI layer that actually consumes lithium,” Biswal said.
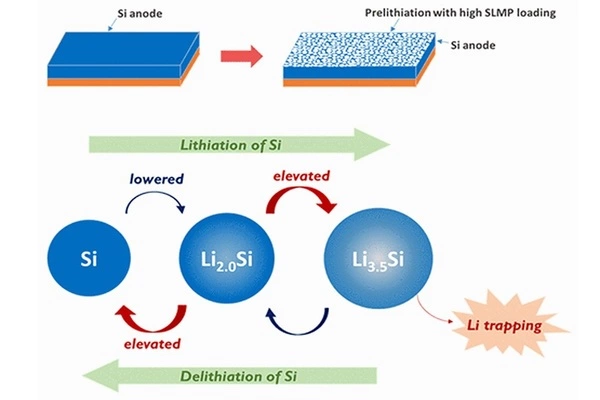
When the electrolyte in a battery cell reacts with electrons and lithium ions, a nanometer-scale layer of salts is deposited on the anode. When the layer is formed, it insulates the electrolyte from the anode, preventing the reaction from continuing. The SEI, on the other hand, can break during subsequent charge and discharge cycles, irreversibly depleting the battery’s lithium reserve.
“The volume of a silicon anode will vary as the battery is cycled, which can break the SEI or otherwise make it unstable,” said Quan Nguyen, the study’s lead author, and a chemical and biomolecular engineering doctoral alum. “We want this layer to remain stable throughout the battery’s later charge and discharge cycles.”
Biswal and her colleagues’ prelithiation method improves SEI layer stability, which means fewer lithium ions are depleted when it is formed.
“Prelithiation is a strategy designed to compensate for the lithium loss that occurs typically with silicon,” Biswal explained. “Think of it like priming a surface, like when you’re painting a wall and need to apply an undercoat first to ensure your paint sticks.” Prelithiation allows us to ‘prime’ the anodes, allowing batteries to be much more stable and have a longer cycle life.”
While these particles and prelithiation are not novel, the Biswal lab was able to improve the process in a way that allows it to be easily integrated into existing battery manufacturing processes.
“One aspect of the process that is definitely new and that Quan developed was the use of a surfactant to help disperse the particles,” Biswal said. “This has not been reported before, and it’s what allows you to have an even dispersion. So instead of them clumping up or building up into different pockets within the battery, they can be uniformly distributed.”
Nguyen explained that mixing the particles with a solvent that lacks a surfactant will result in an uneven coating. Furthermore, spray-coating outperformed other methods of anode application in terms of even distribution.
“The spray-coating method is compatible with large-scale manufacturing,” said Nguyen. Controlling the cell’s cycling capacity is critical to the process. “If you do not control the capacity at which you cycle the cell, a higher amount of particles will trigger this lithium-trapping mechanism that we discovered and described in the paper,” Nguyen explained. “However, if you cycle the cell with an even distribution of the coating, lithium trapping will not occur.”