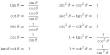Radium tungstate is an inorganic compound of radium, oxygen, and tungsten with the chemical formula RaWO4. This is a salt of wolframic acid and radium. It is typically a white or off-white solid. It is relatively insoluble in water, similar to other tungstates, making it difficult to dissolve in aqueous solutions.
As a compound containing radium, RaWO₄ is radioactive. Radium, being a radioactive element, decays over time, emitting alpha particles. This gives RaWO₄ its characteristic radioactivity, which can pose health risks if handled improperly.
Physical properties
The compound forms a white solid, slightly soluble in water. It is poorly known due to the high radioactivity of radium. It is a radioactive compound with the chemical formula RaWO₄, primarily found in uranium-bearing ores or synthesized in laboratory conditions. Its radioactivity and relatively rare occurrence in nature limit its practical uses, and it is mainly of interest in scientific research related to radiation.
- Chemical formula: O4RaW
- Molar mass: 474 g·mol−1
- Appearance: white solid
- Solubility in water: slightly soluble
Natural Sources
Radium in Uranium Ores: Radium occurs naturally in trace amounts in uranium ores, particularly in minerals like pitchblende (now known as uraninite) and carnotite. These ores contain radium isotopes that can combine with other elements such as tungsten to form tungstates like radium tungstate.
Synthetic Production
Due to the scarcity of radium, RaWO₄ is often synthesized in laboratory conditions. It can be produced by reacting radium salts, such as radium chloride (RaCl₂), with tungsten compounds, typically tungstic acid or tungsten oxides.
Uses
Radium tungstate doesn’t have widespread industrial or commercial applications, but it has been studied in the context of radiochemistry and nuclear physics. Historically, radium compounds were used in luminescent paints and in medical treatments for cancer, although their use has significantly declined due to the harmful effects of radiation.
Safety Concerns
- Radiation Hazard: Radium is highly radioactive and emits harmful radiation, including alpha particles, which can be dangerous if ingested or inhaled. Handling compounds like radium tungstate requires specialized knowledge and safety precautions.
- Health Risks: Prolonged exposure to radium and its compounds can lead to radiation poisoning and increase the risk of cancer, particularly bone cancer, as radium tends to accumulate in bones.