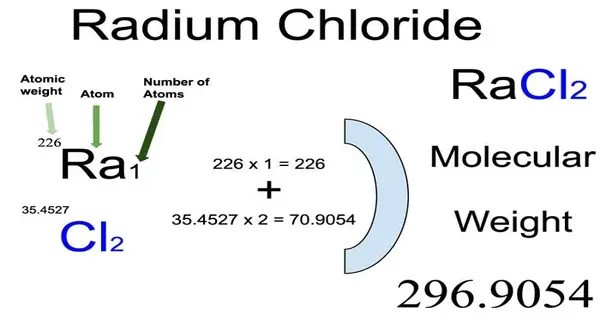
Radium chloride is an inorganic compound with the chemical formula RaCl2. It is a radium salt of hydrogen chloride. It is typically a white, crystalline solid at room temperature, though it can emit a faint, characteristic glow due to its radioactive decay (a phenomenon known as radioluminescence). It is highly radioactive due to the presence of radium isotopes. Radium is a member of the decay series of uranium and thorium, and its radioactivity is a key property.
It was the first radium compound isolated in a pure state. Marie Curie and André-Louis Debierne used it in their original separation of radium from barium. The first preparation of radium metal was by the electrolysis of a solution of this salt using a mercury cathode.
Properties
It is soluble in water and forms a strongly alkaline solution because of the radioactive decay, which produces radon gas (Rn) and other radioactive products. It is relatively stable under normal conditions, but its radioactivity means it poses potential hazards, especially when its isotopes undergo alpha decay.
- Chemical formula: RaCl2
- Molar mass: 296.094 g/mol
- Appearance: Colorless solid, glows blue-green
- Density: 4.9 g/cm3
- Melting point: 900 °C (1,650 °F; 1,170 K)
- Solubility in water: 245 g/L (20 °C)
Preparation
Radium chloride crystallises from aqueous solution as the dihydrate. The dihydrate is dehydrated by heating to 100 °C in air for one hour followed by 5.5 hours at 520 °C under argon. If the presence of other anions is suspected, the dehydration may be effectuated by fusion under hydrogen chloride.
Radium chloride can also be prepared by heating radium bromide in a flow of dry hydrogen chloride gas. It can be produced by treating radium carbonate with hydrochloric acid.
Toxicity
Due to its radioactivity, radium chloride is highly toxic and poses significant health risks if ingested or inhaled. It can lead to radiation poisoning, bone cancer, and other serious health conditions because radium tends to accumulate in the bones.
Occurrence
Radium chloride does not occur naturally in significant amounts as a pure compound. Instead, radium is generally found in trace amounts in uranium and thorium ores, such as pitchblende (now known as uraninite). In the natural decay chain of uranium-238, radium (Ra) is produced through the decay of radon gas. The element can be extracted from ores by chemical processes.
Application
Radium chloride (RaCl₂) is a radioactive compound that was once more widely used but is now primarily of historical and scientific interest due to its significant health risks and the discovery of safer alternatives in medicine and industry.