Boron nitride (BN) is a versatile substance with several engineering and scientific applications. This is largely owing to BN’s intriguing trait of “polymorphism,” which describes its propensity to crystallize into several structures.
This is usually triggered by changes in temperature, pressure, or both. Boron nitride ceramics are utilized in high-temperature equipment and metal casting because of their outstanding thermal and chemical stability. Boron nitride could have use in nanotechnology. Furthermore, although having the same chemical formula, the diverse forms, known as “polymorphs,” have vastly varied physical properties.
As a result, polymorphs are vital in material design, and understanding how to selectively favor the production of the desired polymorph is essential.
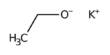
Like carbon, BN exhibits a variety of structural shapes. The most stable structure of BN, hBN (pictured), is isoelectronic with graphite and has the same hexagonal structure as graphite, as well as similar softness and lubricating characteristics. hBN can also be made into nanotubes in the form of graphene-like sheets.
BN polymorphs, on the other hand, are problematic. Despite completing multiple experiments to determine the relative stabilities of BN polymorphs, no consensus has developed.
While computational methods are frequently used to solve these difficulties, BN polymorphs have proven difficult to compute due to the weak “van der Waals (vdW) interactions” between their layers, which are not taken into consideration in these calculations.
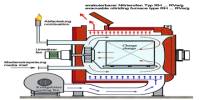
Although a naturally occurring deposit has been discovered, BN is mostly a manufactured material. Pure BN has been attempted since the early twentieth century, but economically suitable versions have only been created in the last 70 years.
Our results reveal that the estimation of relative stabilities is greatly influenced by the exchange correlational functional, or the approximation used in the DFT calculation. As a result, a quantitative conclusion cannot be reached using DFT findings, and a more accurate approach, such as FNDMC, is required.
Kousuke Nakano
Furthermore, the four stable BN polymorphs, rhombohedral (rBN), hexagonal (hBN), wurtzite (wBN), and zinc-blende (cBN), all exhibit within a tight energy range, making capturing minor energy variations combined with vdW interactions much more difficult.
Fortunately, evidence has now been presented by an international study team lead by Assistant Professor Kousuke Nakano of the Japan Advanced Institute of Science and Technology (JAIST).
They used a state-of-the-art first principles computations methodology, namely fixed-node diffusion Monte Carlo (FNDMC) simulations, to solve the problem in their study. The FNDMC is a step in the popular quantum Monte Carlo simulations approach, in which a parametrized many-body quantum “wavefunction” is optimized to achieve the ground state before being fed into the FNDMC.
The scientists also used density functional theory (DFT) and phonon simulations to calculate the Gibbs energy (the useful work available from a system at constant pressure and temperature) of BN polymorphs for various temperatures and pressures. This paper was published in The Journal of Physical Chemistry C and made available online on March 24, 2022.
The most stable structure, according to the FNDMC data, was hBN, followed by rBN, cBN, and wBN. Both at 0 K and 300 K (room temperature), these results were constant.
However, the DFT estimations yielded conflicting results for two different approximations. Dr. Nakano explains these contradictory findings:
“Our results reveal that the estimation of relative stabilities is greatly influenced by the exchange correlational functional, or the approximation used in the DFT calculation. As a result, a quantitative conclusion cannot be reached using DFT findings, and a more accurate approach, such as FNDMC, is required.”
Notably, the FNDMC results were consistent with those obtained using more advanced computation methods like “coupled cluster,” implying that FNDMC is a useful tool for dealing with polymorphs, particularly ones regulated by vdW forces.
When experimental data is lacking, the team demonstrated that it can give other critical information, such as trustworthy reference energies.
Dr. Nakano is optimistic about the method’s future prospects in materials research.
“Our study demonstrates the ability of FNDMC to detect tiny energy changes involving vdW forces, which will stimulate the use of this method for other van der Waals materials,” he says.
“Moreover, molecular simulations based on this accurate and reliable method could empower material designs, enabling the development of medicines and catalysts.”