Lithium-metal (Li-metal) batteries have a lot of potentials to store a lot more energy than current lithium-ion batteries.
A Li-metal electric battery in a car, for example, may go further and have a longer-lasting life than a Li-metal phone battery. However, the metal surface of Li-metal batteries is highly reactive, and they poorly understood the chemistry of these interactions.
Professor Dr. Perla Balbuena of Texas A&M University’s Artie McFerrin Department of Chemical Engineering is employing quantum chemical approaches to track particular reactions on the surfaces of Li-metal batteries. Understanding and forecasting Li-metal battery reactions will improve usability by lowering reactivity.
This study was co-authored by graduate student Dacheng Kuai from Texas A&M’s Department of Chemistry and was just published in the American Chemical Society’s ACS Applied Materials & Interfaces journal.
“We need to understand what type of reactions happen, how to slow down the reactions, what the components are, what the morphology of the evolving products is and how the ions and electrons move through the surface,” said Balbuena. “Understanding these critical issues will allow us to commercialize Li-metal batteries in the near future.”
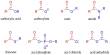
When Li-metal batteries are made, a thin coating called solid-electrolyte interphase (SEI) forms on the anode. Electrolyte breakdown is used to create this film, which is made up of many components.
The chemical composition of the SEI is crucial for ensuring peak performance and increasing the battery’s lifespan. Theoretical predictions can reveal the subtleties of this event at the atomistic and electronic levels through experimental efforts.
This research can be a driving force for batteries in a greener, more efficient direction. I know that this work will be helpful 10 years from now because 10 years ago, we made our initial contributions on Li-ion batteries and our findings helped on the development of today’s successful technology. It is a cycle of continuous improvement.
Dr. Perla Balbuena
The researchers focused on a polymer that forms on the battery’s inside surfaces as a result of electrolyte reactions. It’s difficult to pinpoint this precise polymer reaction, yet it’s important to improve the SEI.
The researchers mapped a time development of the polymer formation reaction by simulating the interface at the atomic level and solving correct quantum chemical equations.
“What differentiates this research is starting from the microscopic-level description and letting the system evolve according to its electronic redistribution upon chemical reaction,” Balbuena said.
“There are many experimental techniques that can follow and monitor the reactions, but they’re challenging. With this simulation, we can get new insights. We isolate the part of the system that is responsible for important chemical events. We follow that specific group of molecules and analyze the reactions spontaneously occurring at the surface of electrodes.”
The computational tools utilized in this study are unique in that they can calculate the least energy configurations and the arrangement of the molecules during the reaction, allowing them to monitor the reaction from start to finish.
The SEI species polymerizing could be good for Li-metal batteries, according to the researchers, because they can help manage the reactivity of the battery components.
“We are pleased about the results, as they provide insight into what could happen when using real electrodes,” said Balbuena.
These studies show how computational techniques can help create batteries that are more environmentally friendly, have longer lifespans, and are less expensive to manufacture. Balbuena expects that the approaches she discovered in her research will be useful for years to come as better chemistries emerge.
“This research can be a driving force for batteries in a greener, more efficient direction,” she said. “I know that this work will be helpful 10 years from now because 10 years ago, we made our initial contributions on Li-ion batteries and our findings helped on the development of today’s successful technology. It is a cycle of continuous improvement.”