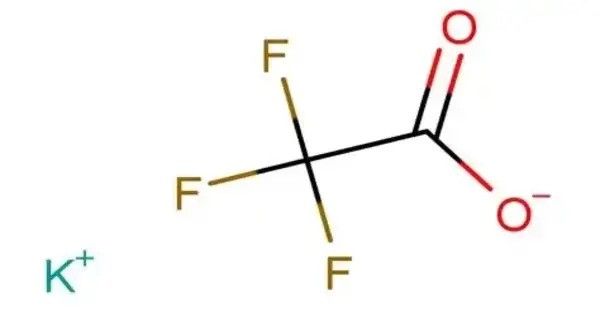
Potassium trifluoroacetate is the trifluoroacetate salt of potassium, with the chemical formula CF3COOK. It can form an acid salt KH(CF3COO)2. It appears as a white to off-white crystalline solid and is highly soluble in water and polar organic solvents. The presence of three electronegative fluorine atoms in the trifluoromethyl group imparts significant electron-withdrawing character, making it useful in various organic synthesis applications.
It serves primarily as a reagent in fluorination reactions, decarboxylations, and oxidative transformations. In synthesis, it can be used to generate trifluoromethylated intermediates, making it valuable in pharmaceuticals and agrochemical development due to the metabolic stability and lipophilicity introduced by the CF₃ group. Its versatility and role in introducing fluorinated motifs make potassium trifluoroacetate a useful building block in modern synthetic chemistry.
Preparation
Potassium trifluoroacetate can be obtained by reacting trifluoroacetic acid with potassium hydroxide, potassium carbonate or potassium bicarbonate.
CF3COOH + KOH → CF3COOK + H2O
It can decompose when heated and reaches the maximum decomposition rate at 220 °C. The products are potassium fluoride and some volatile products, such as carbon dioxide, carbon monoxide, trifluoroacetyl fluoride, etc.
Properties
It is generally considered stable under normal conditions but should be handled with standard laboratory precautions, as fluorinated compounds can be reactive under certain conditions. Proper storage in a cool, dry place is recommended.
- Chemical formula: CF3COOK
- Melting point: 135–137 °C (408–410 K)
- Boiling point: 145 °C (418 K)
- Appearance: White crystalline solid
- Odor: Odorless
- Solubility: Highly soluble in water, alcohols, and polar organic solvents
- Stability: Hygroscopic, stable under dry conditions
- pKa (of parent acid): ~0.5 (strong acid – trifluoroacetic acid)
Synthesis
Synthetic compound — does not occur naturally
Usually prepared by neutralizing trifluoroacetic acid with potassium hydroxide (KOH):
CF3COOH + KOH → CF3COOK+H2O
Available commercially in pure or reagent grade
Safety and Handling
- Irritant: Can cause skin and eye irritation
- Hygroscopic: Must be stored in a dry, sealed container
- Use gloves and goggles during handling; work in a fume hood if heating or reacting
Applications
- Organic synthesis (especially trifluoromethylation)
- Used in medicinal chemistry and materials science where fluorinated compounds are desired
- Employed in electrochemical CF₃ sources for synthesis of fluorinated drugs and agrochemicals