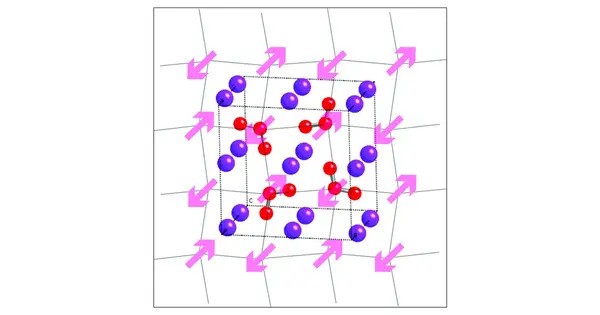
Potassium ozonide is an oxygen rich compound of potassium. It is relatively unstable and decomposes upon heating or when exposed to moisture. The compound breaks down to release oxygen and potassium oxide (K₂O), as well as other oxygen species. It is an ozonide, meaning it contains the ozonide anion (O3−). In polarized light, it shows pleochroism. Hybrid functional calculations have predicted the compound is an insulator with a band gap of 3.0 eV, and has magnetic behavior which departs from the Curie–Weiss law.
Properties
- Chemical formula: KO3
- Molar mass: 87.10 g/mol
- Appearance: Red crystalline solid
- Density: 1.990 g/cm3
- Melting point: 25 °C (77 °F; 298 K) (decomposes)
- Solubility in water: Reacts
- Solubility: Soluble in liquid anhydrous ammonia
- Refractive index (nD): 1.39
Reaction
The compound can be created by reacting ozone with potassium hydroxide, but the yield is quite low, only 5-10%.
6 KOH + 4 O3 → 4 KO3 + 2 KOH(H2O) + O2
The compound is metastable, and will decompose to potassium superoxide and oxygen, especially if there is any water in the atmosphere. Long-term storage in very dry atmosphere is possible below around 0 °C.
KO3 → KO2 + ½ O2
This compound reacts with water to form potassium hydroxide and potassium superoxide.
Laboratory Preparation: Potassium ozonide is typically synthesized in laboratories under controlled conditions, usually by reacting potassium metal with ozone (O₃) at low temperatures. This reaction typically occurs in a solid state under anhydrous conditions.
The reaction for preparation is:
2K + 3O3 → 2KO3
Industrial Uses
While potassium ozonide does not have widespread commercial uses, it may be used in specialized reactions where high concentrations of ozone or its derivatives are needed. Ozonides, including KO₃, can be used in organic chemistry for certain oxidation reactions or in ozone-related processes.
Natural Occurrence
Potassium ozonide is not typically found in nature in significant amounts, as it is relatively unstable and would decompose in natural environments. Ozone itself occurs naturally in the Earth’s stratosphere, but it is not commonly found as a stable compound in mineral form. Ozone can be generated in specific natural phenomena, like lightning, but the stable ozonides formed with metals are mostly a product of controlled laboratory conditions.