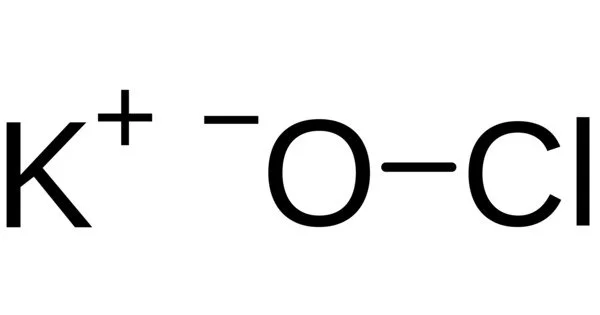The potassium salt of hypochlorous acid is potassium hypochlorite (chemical formula KClO). It is used in varying concentrations and is frequently diluted in water solution. It is light grey in color and has a strong chlorine odor. It can be used to disinfect. Its solution is a colorless aqueous solution with an unpleasant odor of chlorine.
Potassium hypochlorite was first synthesized in 1789 by Claude Louis Berthollet in his laboratory in Javel, Paris, France, by passing chlorine gas through a potash lye solution. The resulting liquid, dubbed “Eau de Javel” (“Javel water”), was a weak potassium hypochlorite solution. Due to manufacturing difficulties, the product was modified to use sodium instead of potassium, giving rise to sodium hypochlorite, which is now widely used as a disinfectant.
Properties
- Chemical formula: KClO
- Molar mass: 90.55 g/mol
- Appearance: light grey liquid
- Odor: Chlorine-like
- Density: 1.160 g/cm3
- Melting point: −2 °C (28 °F; 271 K)
- Boiling point: 102 °C (216 °F; 375 K) (decomposes)
- Solubility in water: 25%

Preparation
Chlorine and potassium hydroxide can be used to make potassium hypochlorite. It can also be created through the electrolysis of potassium chloride solution. To avoid the formation of potassium chlorate, the reaction mixture must be kept cold in both methods.
The reaction of chlorine with a potassium hydroxide solution produces potassium hypochlorite:
Cl2 + 2 KOH → KCl + KClO + H2O
This is the traditional method, first used by Claude Louis Berthollet in 1789.
Another production method is electrolysis of potassium chloride solution. With both methods, the reaction mixture must be kept cold to prevent formation of potassium chlorate.
Uses
Potassium hypochlorite is used to disinfect drinking water as well as sanitize surfaces. Because its degradation produces potassium chloride rather than sodium chloride, it has been promoted for use in agriculture where potassium addition to soil is desired.
- Used for disinfecting water and sanitizing the surface
- Used as a bleaching agent
- Used as an industrial oxidizer
- Used in agriculture field
Safety and toxicology
Potassium hypochlorite, like sodium hypochlorite, is an irritant. When it comes into contact with the skin, eyes, or mucous membranes, it can cause severe damage. Bronchial irritation, difficulty breathing, and, in severe cases, pulmonary edema can result from inhaling a KClO mist. Strong concentrations can be lethal if consumed.
Potassium hypochlorite is not a fire or explosive hazard on its own. It can, however, react violently with a variety of chemicals, including urea, ammonium salts, methanol, acetylene, and many organic compounds. Toxic chlorine gas can be produced by heating and acidification.