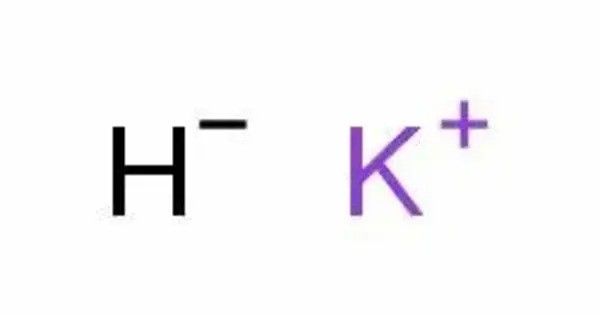
Potassium hydride, KH, is the inorganic compound of potassium and hydrogen. It is an alkali metal hydride. It is a strong ionic compound and is part of the alkali metal hydrides, which are known for their highly reactive nature. It is a white solid, although commercial samples appear gray.
KH is highly soluble in water, but it reacts violently with water, producing potassium hydroxide (KOH) and hydrogen gas (H₂). This reaction is exothermic and can be explosive. It is a powerful superbase that is useful in organic synthesis. It is sold commercially as a slurry (~35%) in mineral oil or sometimes paraffin wax to facilitate dispensing.
Preparation
Potassium hydride is produced by direct combination of the metal and hydrogen at temperatures between 200 and 350 °C:
2 K + H2 → 2 KH
This reaction was discovered by Humphry Davy soon after his 1807 discovery of potassium, when he noted that the metal would vaporize in a current of hydrogen when heated just below its boiling point.
Potassium hydride is soluble in fused hydroxides (such as molten sodium hydroxide) and salt mixtures, but not in organic solvents.
Properties
- Chemical formula: KH
- Molar mass: 40.1062 g/mol
- Appearance: white to gray crystalline powder
- Density: 1.43 g/cm3
- Melting point: decomposes at ~400 °C
- Solubility in water: reacts
- Solubility: insoluble in benzene, diethyl ether, carbon disulfide
Reactions
KH reacts with water according to the reaction:
KH + H2O → KOH + H2
As a superbase, potassium hydride is more basic than sodium hydride. It is used to deprotonate certain carbonyl compounds to give enolates. It also deprotonates amines to give the corresponding amides of the type KNHR and KNR2.
Occurrences
Potassium hydride is not commonly found in nature in its pure form because it is highly reactive. Instead, it is typically produced synthetically in laboratories or industrial settings through reactions such as the following:
Synthesis
Direct reaction: KH can be synthesized by reacting potassium metal with hydrogen gas at high temperatures:
2K + H2 → 2KH
Inert atmosphere: This reaction usually occurs under an inert atmosphere (like argon or nitrogen) to prevent the potassium metal from reacting with moisture in the air.
Natural Occurrence
While potassium hydride itself doesn’t occur naturally, potassium is found abundantly in the Earth’s crust in various mineral forms, such as potash (K₂CO₃), sylvite (KCl), and carnallite (KMgCl₃·6H₂O). However, potassium hydride must be produced artificially because of its reactivity.
Applications
Due to its strong reactivity, potassium hydride is mainly used in specialized chemical and industrial applications, including:
- Reducing agent in organic synthesis, especially in reactions requiring the removal of protons (deprotonation).
- Desiccant for drying organic solvents, as it can react with trace amounts of moisture.
- Preparation of potassium-based compounds, such as potassium hydroxide and potassium amides.
Safety
KH can be pyrophoric in air, react violently with acids, and can ignite upon contact with oxidants. As a suspension in mineral oil, KH is less dangerous.