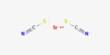
Potassium citrate (K₃C₆H₅O₇) is a potassium salt of citric acid, appearing as a white, crystalline powder with a slightly saline taste. It is a white, hygroscopic crystalline powder. It is odorless with a saline taste. It is highly soluble in water and slightly hygroscopic. It contains 38.28% potassium by mass. In the monohydrate form, it is highly hygroscopic and deliquescent. Chemically, it consists of three potassium ions bonded to citrate, an organic acid found naturally in citrus fruits.
As a food additive, potassium citrate is used to regulate acidity, and is known as E number E332. Medicinally, it may be used to control kidney stones derived from uric acid or cystine. In 2020, it was the 297th most commonly prescribed medication in the United States, with more than 1 million prescriptions.
Medically, potassium citrate is used as an alkalinizing agent to reduce urine acidity, helping prevent kidney stones (especially uric acid and calcium oxalate types) and manage gout. By increasing urinary pH and citrate levels, it reduces crystal formation in the urinary tract. It’s also prescribed to treat potassium deficiency (hypokalemia) when dietary intake is insufficient. Its stability, low toxicity, and versatile properties make potassium citrate valuable in medicine, food processing, and laboratory applications.
Properties
- Chemical formula: K3C6H5O7
- Molar mass: 306.395 g/mol
- Appearance: white powder hygroscopic
- Odor: odorless
- Density: 1.98 g/cm3
- Melting point: 180 °C (356 °F; 453 K)
- Boiling point: 230 °C (446 °F; 503 K)
- Solubility in water: soluble
- Solubility: soluble in glycerine, insoluble in ethanol (95%)
- Acidity (pKa): 8.5
Synthesis
Potassium citrate can be synthesized by the neutralization of citric acid which is achieved by the addition of potassium bicarbonate, potassium carbonate or potassium hydroxide to it. The solution can then be filtered and the solvent can be evaporated till granulation.
Occurrences
Potassium citrate is not typically found in nature in pure form but occurs as a natural component in certain fruits and vegetables, especially citrus fruits such as lemons, limes, and oranges. It is naturally formed in plants as part of their metabolic processes involving citric acid. Commercially, potassium citrate is produced by neutralizing citric acid with potassium hydroxide or potassium carbonate. It is widely used in the food industry as an acidity regulator, in medicine for urinary alkalinization, and in beverages for flavor enhancement and electrolyte replenishment.
Uses
Potassium citrate is rapidly absorbed when given by mouth, and is excreted in the urine. Since it is an alkaline salt, it is effective in reducing the pain and frequency of urination when these are caused by highly acidic urine. It is used for this purpose in dogs and cats, but is chiefly employed as a non-irritating diuretic.
Safety
Potassium citrate is generally safe when used as directed, but excessive intake can cause hyperkalemia (high potassium levels), leading to muscle weakness, heart rhythm disturbances, or even cardiac arrest. Therefore, dosage must be carefully monitored, especially in patients with kidney disease or on potassium-sparing medications.