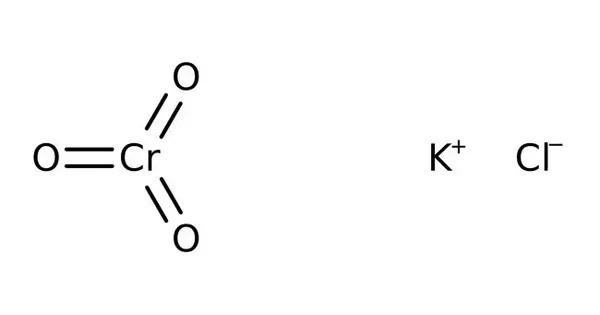
Potassium chlorochromate (KCrO₃Cl) is an orange crystalline inorganic compound containing potassium, chromium in the +6 oxidation state, oxygen, and chlorine. It is a water-soluble orange compound is used occasionally for oxidation of organic compounds. It is typically prepared by reacting potassium dichromate (K₂Cr₂O₇) with concentrated hydrochloric acid, resulting in the replacement of one oxygen atom by a chlorine atom in the chromate structure. It is sometimes called Péligot’s salt, in recognition of its discoverer Eugène-Melchior Péligot.
Properties
It is moderately soluble in water and stable under dry conditions but can decompose in the presence of moisture or reducing agents. As a hexavalent chromium compound, it is a strong oxidizing agent and highly toxic, posing environmental and health hazards, including carcinogenicity. Its bright orange color and crystalline nature make it identifiable, but its oxidizing power and corrosiveness demand caution in laboratory work.
- Chemical formula: KCrO3Cl
- Molar mass: 174,5472 g/mol
- Appearance: orange solid
- Density: 2.5228 g/cm3
- Solubility in water: Soluble[vague]
- Odor: Odorless
- Melting point: Decomposes before melting
- Oxidation state of Cr: +5
- Stability: Sensitive to heat and light; decomposes to chromium(VI) and chromium(III) compounds
- Reactivity: Strong oxidizing agent; reacts with reducing agents and some organic compounds
Structure and synthesis
Potassium chlorochromate was originally prepared by treating potassium dichromate with hydrochloric acid. An improved route involves the reaction of chromyl chloride and potassium chromate:
K2CrO4 + CrO2Cl2 → 2KCrO3Cl
The salt consists of the tetrahedral chlorochromate anion. The average Cr=O bond length is 159 pm, and the Cr-Cl distance is 219 pm.
Reactions
Although air-stable, its aqueous solutions undergo hydrolysis in the presence of strong acids. With concentrated hydrochloric acid, it converts to chromyl chloride, which in turn reacts with water to form chromic acid and additional hydrochloric acid. When treated with 18-crown-6, it forms the lipophilic salt [K(18-crown-6)]CrO3Cl.
Peligot’s salt can oxidize benzyl alcohol, a reaction which can be catalyzed by acid. A related salt, pyridinium chlorochromate, is more commonly used for this reaction.
Occurrences
Potassium chlorochromate does not occur naturally. It is synthetically prepared in laboratories by reacting potassium dichromate (K₂Cr₂O₇) with hydrochloric acid (HCl) under controlled conditions:
𝐾2𝐶𝑟2𝑂7 + 2𝐻𝐶𝑙 → 2KCrO3Cl + H2O
Application
Potassium chlorochromate has been used in organic synthesis, particularly for selective oxidation of alcohols to carbonyl compounds, similar to pyridinium chlorochromate (PCC) but in inorganic form. It is also used in some analytical chemistry applications involving chromium-based oxidation. It is mainly used:
- As an oxidizing agent in organic chemistry (e.g., oxidation of alcohols to aldehydes/ketones)
- In analytical chemistry for certain qualitative tests
- Occasionally in laboratory demonstrations for Cr(V) compounds
Due to its toxicity and environmental concerns, its use has declined in favor of less hazardous oxidants. Proper handling requires gloves, goggles, fume hoods, and strict waste disposal measures to prevent chromium(VI) contamination of water and soil.
Safety
Potassium chlorochromate is toxic upon ingestion, and may cause irritation, chemical burns, and even ulceration on contact with the skin or eyes. Like other hexavalent chromium compounds, it is also carcinogenic and mutagenic.