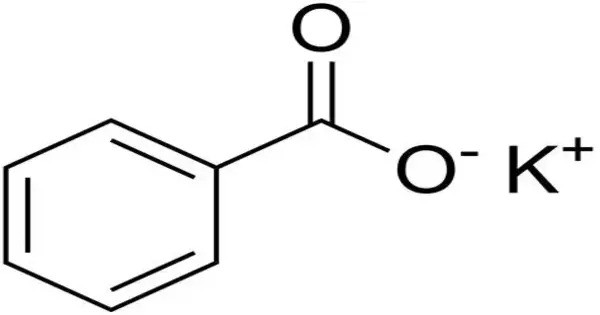
Potassium benzoate (E212), the potassium salt of benzoic acid, is a food preservative that inhibits the growth of mold, yeast and some bacteria. It is a white, odorless powder used primarily as a food preservative to extend shelf life. It works best in low-pH products, below 4.5, where it exists as benzoic acid. It’s the potassium salt of benzoic acid, commonly found in acidic foods like soft drinks, fruit juices, and sauces, where it inhibits the growth of bacteria, yeasts, and molds.
Acidic foods and beverages such as fruit juice (citric acid), sparkling drinks (carbonic acid), soft drinks (phosphoric acid), and pickles (vinegar) may be preserved with potassium benzoate. It is approved for use in most countries including Canada, the United States and the European Union, where it is designated by the E number E212.
Potassium benzoate is also used in the whistle in many fireworks.
Properties
Potassium benzoate is water-soluble, making it easy to incorporate into liquid products. Unlike sodium benzoate, it’s preferred in low-sodium foods due to its potassium content. However, excessive consumption may lead to mild side effects like allergic reactions in sensitive individuals. It’s also used in pharmaceuticals and cosmetics for similar preservative purposes.
- Chemical formula: C7H5KO2
- Molar mass: 160.213 g·mol−1
- Appearance: White hygroscopic solid
- Odor: Odorless
- Density: 1.5 g/cm3
- Melting point: >300 °C (572 °F; 573 K)
- Solubility in water: 69.87 g/100 mL (17.5 °C), 88.33 g/100 mL (50 °C)
- Solubility in other solvents: Soluble in ethanol, Slightly soluble in methanol
- Insoluble in ether
Synthesis
One very common way to make potassium benzoate is by oxidizing toluene to benzoic acid followed by a neutralization with potassium hydroxide:
C6H5COOH + KOH → C6H5COOK + H2O
Another way to synthesize potassium benzoate in the lab setting is by hydrolyzing methyl benzoate with potassium hydroxide:
C6H5COOCH3 + KOH → C6H5COOK + CH3OH
Chemically, it’s produced by neutralizing benzoic acid with potassium hydroxide. Its stability and effectiveness make it a popular choice, though some debate its use due to potential benzene formation in certain conditions, particularly when combined with ascorbic acid under heat or light exposure. Always store products containing it properly to minimize risks.
Reactions
Potassium benzoate, like sodium benzoate, can be decarboxylated with a strong base and heat:
C6H5COOK + KOH → C6H6 + K2CO3
Mechanism of food preservation
The mechanism of food preservation begins with the absorption of benzoic acid into the cell. If the intracellular pH changes to 5 or lower, the anaerobic fermentation of glucose through phosphofructokinase is decreased by 95%.
Applications
Potassium benzoate does not occur naturally in significant amounts but is synthetically produced for industrial and food applications. Its occurrences are primarily tied to its use as an additive:
Food Industry:
- Used as a preservative in acidic foods and beverages, such as soft drinks, fruit juices, jams, and pickles, to extend shelf life by preventing microbial spoilage.
- Often preferred over sodium benzoate in low-sodium food products due to its potassium content.
Cosmetics and Pharmaceuticals: Found in some cosmetic products (e.g., creams, lotions) and pharmaceuticals as a preservative to prevent microbial contamination.
Industrial Applications: Occasionally used in chemical formulations or as a corrosion inhibitor in certain industrial processes.
Natural Context: While benzoic acid occurs naturally in some fruits (e.g., cranberries, plums) and spices, potassium benzoate itself is not naturally occurring and is synthesized by neutralizing benzoic acid with potassium hydroxide.
Safety and health
Potassium benzoate has low acute toxicity upon oral and dermal exposure. The Food Commission, which campaigns for safer, healthier food in the UK, describes potassium benzoate as “mildly irritant to the skin, eyes and mucous membranes”.