Polycarbonate
Definition
Polycarbonate is a synthetic thermoplastic resin, a linear polymer of carbonic acid, used for molded products, films, and nonbreakable windows. The main advantage of this material over other types of plastic is its great strength combined with light weight. While acrylic is 17% stronger than glass, polycarbonate is nearly unbreakable. Bulletproof windows and enclosures as seen inside banks or at drive-throughs are often made of this plastic. Add to this the advantage that it is just 1/3 the weight of acrylic, or 1/6 as heavy as glass, and the only drawback is that it is more expensive than either.
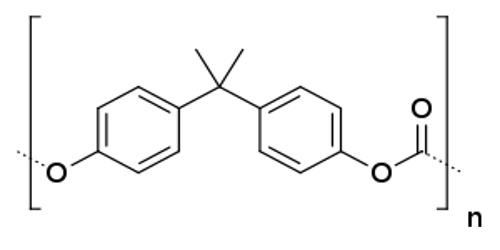
This is a polyester made by the mixing of phenyl groups, carbonate groups and two methyl groups, and is formed by the reaction of carbonyl chloride and bisphenol A in an interfacial process. Polycarbonates do not have a unique resin identification code (RIC) and are identified as “Other”, 7 on the RIC list. Products made from polycarbonate can contain the precursor monomer bisphenol A (BPA).
Compact disks and digital versatile discs (DVDs) are perhaps the most readily recognized examples of polycarbonate. Anyone who has archived files on a writable CD, then later tried to break it before throwing it away, knows just how tough this material can be.
Structure and Properties of Polycarbonate
Polycarbonate (PC)/modified clay nanocomposites were prepared, in the absence and presence of different amounts of maleic anhydride grafted polypropylene (PP-g-MA), by direct melt blending. Their structures, as well as mechanical, morphological and thermal properties, were characterized by X-ray diffractometry (XRD), tensile testing, transmission electron microscopy (TEM), and thermogravimetric analysis (TGA).
Polycarbonates received their name because they are polymers containing carbonate groups (−O−(C=O)−O−). A balance of useful features, including temperature resistance, impact resistance and optical properties, positions polycarbonates between commodity plastics and engineering plastics.

Polycarbonate is similar to acrylic and has glass properties: it is transparent and has better light transmission than glass. Products made from this material have the following qualities:
- High impact and fracture resistance
- Better heat resistance
- It is relatively light (half that of glass); it is easy to handle
- Machinable and easy to fabricate to meet special needs
- It has a high refractive index
- Durable with excellent toughness
Polycarbonate is stable over a range of temperatures with a glass transition temperature of about 297°F (147°C) and flows at about 311 °F (155 °C). It can be affected by scratch, UV rays and chemicals, but the addition of special additives can improve the resistance of the plastic against the constraints. Products made from polycarbonate go through two dominant processes: extrusion and injection molding.
Uses of Polycarbonate
Clear polycarbonate is used to make eyeglasses because of its excellent transparency, durability, and high refractive index. This means that it bends light to a far greater degree than glass or other plastics of equal thickness. Polycarbonate lenses are also used in quality sunglasses that incorporate filters to block ultra-violet (UV) rays and near-UV rays. The lenses can also be polarized to block glare, and their high impact resistance makes them perfect for sports.
The electronics industry also uses polycarbonate. It has been used to create transparent colored computer cases, for example, and many cell phones, pagers, and laptops also use it in their casings.
Other uses for polycarbonate include greenhouse enclosures, automobile headlights, outdoor fixtures, and medical industry applications, though the list is virtually endless. Somewhat less toxic than polyvinyl chloride (PVC) to produce, this plastic nevertheless requires toxic chemicals in its production phase. It is, however, recyclable and environmentally preferable to PVC in applications for which either material can be used.
Reference: