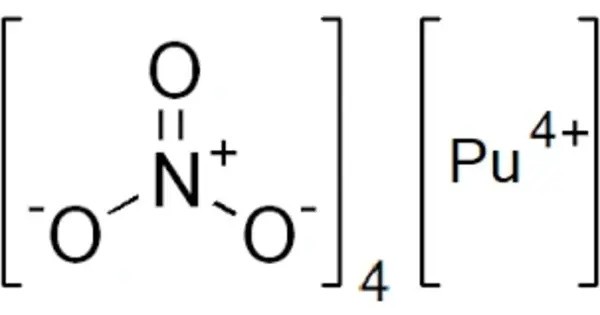Plutonium nitride is a binary inorganic compound of plutonium and nitrogen with the chemical formula PuN. It is a chemical compound consisting of plutonium and nitrogen. It’s primarily of interest in the field of nuclear science and technology. It can be synthesized by direct reaction of plutonium metal with nitrogen gas at high temperatures. Alternatively, it can be produced by the carbothermic reduction of plutonium dioxide (PuO₂) in the presence of nitrogen.
Synthesis
Plutonium nitride can be prepared by the reaction of plutonium hydrides with nitrogen or ammonia at a temperature of 650 °C and a pressure of 0.3 kPa.
Another method to prepare plutonium nitride is from the reduction of plutonium(III) iodide with sodium in liquid ammonia:
PuI3 + NH3 + 3Na → PuN + 3NaI
Other reactions can also produce plutonium nitride:
Pu + 1/2N2 +3/2H2 → PuN + 3/2H2 (at 1000°C)
PuCl3 + 1/2N2 +3/2H2 → PuN + 3HCl (at 800–900°C)
Physical properties
Plutonium nitride forms grey crystals in the cubic system with Fm3m space group. It can be dissolved electrochemically.
- Chemical formula: NPu
- Molar mass: 258 g·mol−1
- Appearance: Grey crystalline solid
- Density: 14.2 g/cm3
- Melting point: 2,589 °C (4,692 °F; 2,862 K)
- Solubility in water: insoluble
Uses
Plutonium nitride can be used as fuel in nuclear reactors.
- Nuclear Fuel: One of the primary uses of plutonium nitride is as a nuclear fuel. It has potential advantages over traditional oxide fuels, such as higher thermal conductivity and better performance at high temperatures.
- Research: It is used in research settings to study the properties of plutonium and the behavior of nuclear fuels.
Safety and Handling
- Radioactivity: Like all plutonium compounds, PuN is highly radioactive and must be handled with extreme care.
- Toxicity: Plutonium compounds are highly toxic, and exposure can pose serious health risks, including cancer.