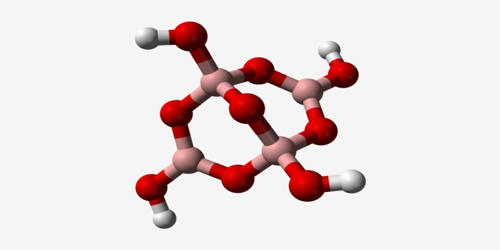
Phosphorus selenides are a relatively obscure group of compounds. There have been some studies of the phosphorus – selenium phase diagram and the glassy amorphous phases are reported. They typically exist in various stoichiometric ratios and can exhibit different structures and properties depending on the specific compound.
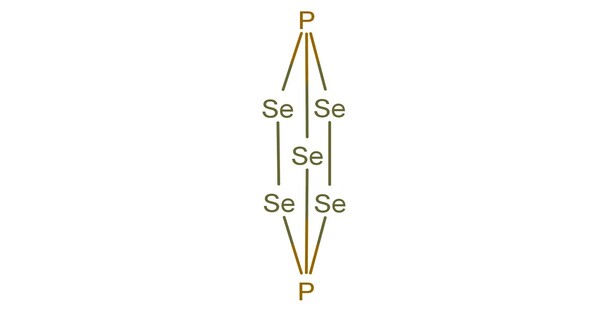
The compounds that have been reported are shown below. While some of phosphorus selenides are similar to their sulfide analogues, there are some new forms, molecular P2Se5 and the polymeric catena-[P4Se4]x. There is also some doubt about the existence of molecular P4Se10.
Types
- Phosphorus(III) selenide (P2Se3): This compound is known for its dark red color and is a solid at room temperature. It has a molecular structure where phosphorus and selenium are bonded in a 2:3 ratio.
- Phosphorus(V) selenide (P4Se3): This compound is a yellow solid and has a different structure compared to P2Se3. The phosphorus to selenium ratio is different in this compound.
- Phosphorus selenide (PSe): This is a less common compound, and its chemistry is less well-studied compared to the others.
Reactivity
Phosphorus selenides are generally reactive. They can hydrolyze in the presence of water to form phosphorous acid or phosphoric acid and selenium dioxide. They also react with acids and bases, producing corresponding selenides or phosphates.
Electronic and Optical Properties
The electronic properties of phosphorus selenides can vary based on their specific structure. Some compounds exhibit semiconducting properties, which makes them interesting for certain applications in electronics.
Synthetic Preparation
Phosphorus selenides are typically synthesized in the laboratory. They can be prepared by direct reaction of phosphorus and selenium under controlled conditions. They are not found in significant amounts in nature but are of interest in chemical research.
Natural Occurrences
While phosphorus selenides are not commonly found in nature, selenium is an element that occurs in several minerals and ores. Phosphorus and selenium can both be present in trace amounts in various geological formations. However, specific phosphorus selenides are not usually isolated from natural sources.
Applications
Due to their unique properties, phosphorus selenides have some specialized applications. They are used in research contexts, including studies related to semiconductors and materials science. They may also be involved in the synthesis of other compounds or materials.