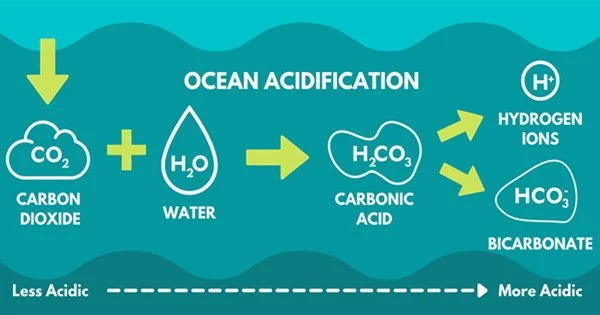
Ocean acidification is a process by which the pH of seawater becomes more acidic over time. This occurs when carbon dioxide (CO2) from the atmosphere dissolves in seawater and reacts with water molecules to form carbonic acid. This reaction releases hydrogen ions, which lower the pH of seawater and make it more acidic.
The process of ocean acidification is a major concern because it can have significant impacts on marine ecosystems, including marine life, food webs, and nutrient cycling. As seawater becomes more acidic, it can become more difficult for organisms to build and maintain their shells and skeletons, which are made of calcium carbonate. This can lead to reduced growth, reproduction, and survival of marine species, especially those that rely on these structures for protection or for accessing food.
Ocean acidification is the decrease in the pH value of the Earth’s ocean. Between 1751 and 2021, the average pH of the ocean surface fell from approximately 8.25 to 8.14. Carbon dioxide emissions from human activities are the primary cause of ocean acidification, with atmospheric carbon dioxide (CO2) levels exceeding 410 ppm (in 2020). The oceans absorb CO2 from the atmosphere. This results in the formation of carbonic acid (H2CO3), which dissociates into a bicarbonate ion (HCO3) and a hydrogen ion (H+). Free hydrogen ions (H+) lower the pH of the ocean, increasing acidity (this does not mean that seawater is acidic yet; it is still alkaline, with a pH higher than 8).
The concentration of carbonate ions, which are the main building blocks for calcium carbonate (CaCO3) shells and skeletons, decreases as pH decreases. Marine calcifying organisms, such as mollusks, oysters, and corals, are especially vulnerable because they rely on calcium carbonate to build shells and skeletons.
In addition to affecting individual species, ocean acidification can also affect entire ecosystems. For example, changes in the growth and survival of planktonic species can have cascading effects on the entire food web, as these organisms form the base of many marine ecosystems. Changes in ocean chemistry can also affect nutrient cycling and the availability of certain nutrients, which can in turn affect the growth and distribution of marine species.
The primary driver of ocean acidification is the increase in atmospheric CO2 concentrations caused by human activities such as burning fossil fuels and deforestation. To mitigate the impacts of ocean acidification, it is important to reduce greenhouse gas emissions and to promote sustainable practices that protect marine ecosystems.
The increase in pH from 8.25 to 8.14 represents a nearly 30% increase in hydrogen ion concentration in the world’s oceans. The pH and carbonate saturation states of the sea surface vary depending on ocean depth and location. Colder and higher latitude waters are capable of absorbing more CO2. This can cause acidity to rise, lowering pH and carbonate saturation levels in these areas.
Other factors that influence the atmosphere-ocean CO2 exchange, and thus local ocean acidification, are ocean currents and upwelling zones, proximity to large continental rivers, sea ice coverage, and atmospheric exchange with nitrogen and sulfur from fossil fuel combustion and agriculture.
Decreased ocean pH has a range of potentially harmful effects for marine organisms. Reduced calcification, decreased metabolic rates, decreased immune responses, and decreased energy for basic functions such as reproduction are all examples. Ocean acidification is thus affecting marine ecosystems, which provide food, livelihoods, and other ecosystem services to a large portion of humanity.