Nickel(II) sulfate is an inorganic compound with the formula NiSO4(H2O)6. Depending on the degree of hydration, it can take the form of greenish-yellow, blue, or green crystals. This highly soluble blue-green salt is a popular source of Ni2+ ions in electroplating. It’s used to nickel-plate iron and copper, as a catalyst, a mordant in the textile industry, and as a coating for other things.
Nickel sulfate occurs naturally as retgersite, a rare mineral. The most serious danger is the threat to the environment. Immediate action should be taken to limit its environmental spread.
Properties
Nickel sulphate is an odorless soluble nickel salt that appears as blue to blue-green transparent crystals. Strong acids are incompatible with it. It has numerous industrial applications, including nickel patch testing, nickel plating, as a raw material for catalyst production, dyeing and printing fabrics as a mordant, blackening zinc and brass, and jewelry manufacturing.
- Melting point: 848°C
- Density: 3.68
- Form: green-yellow orthorhombic crystals
- Water Solubility: 27.3-27.7 g/100 mL at 20 ºC
- Boiling Point: 840 °C
- Appearance: blue crystals
- Molar Mass: 154.75 g/mol

Structures
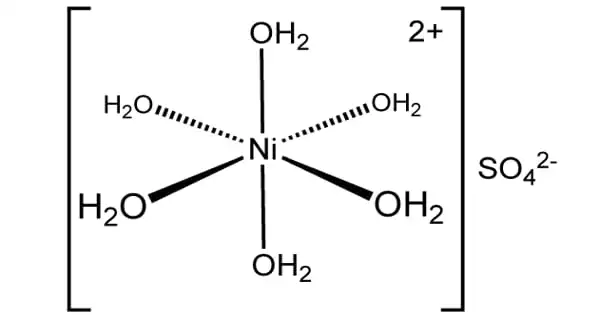
There are at least seven nickel(II) sulfate salts known. The hydration or crystal habit of these salts differs. Between 30.7 and 53.8 °C, the common tetragonal hexahydrate crystallizes from an aqueous solution. A heptahydrate crystallizes below these temperatures, and an orthorhombic hexahydrate crystallizes above these temperatures. NiSO4 in its yellow anhydrous form is a high melting solid that is rarely encountered in laboratories. The hydrates are heated above 330 °C to produce this material. At even higher temperatures, it decomposes to nickel oxide.
X-ray crystallography measurements show that NiSO4·6H2O consists of the octahedral [Ni(H2O)6]2+ ions. These ions in turn are hydrogen-bonded to sulfate ions. Dissolution of the salt in water gives solutions containing the aquo complex [Ni(H2O)6]2+.
All nickel sulfates are paramagnetic.
Preparation
There are several methods for producing nickel sulfate. It is made by dissolving nickel metal, oxide, or carbonate in sulfuric acid. Powdered metal or black nickel oxide is added to hot dilute sulfuric acid in such methods, or nickel carbonate is added to dilute sulfuric acid at room temperature:
NiO + H2SO4 → NiSO4 + H2O
NiCO3 + H2SO4 → NiSO4 + CO2 + H2O
Impurities may be precipitated by treating the diluted solution with barium carbonate. Evaporation followed by cooling crystallizes hexahydrate in two modifications: blue tetragonal crystals obtained between 31.5 and 53.3°C, and above 53.3°C green monoclinic crystals form. The heptahydrate, NiSO4•7H2O, crystallizes at ordinary temperatures from pure aqueous solutions.
Nickel sulfate also can be produced on a large-scale by gas-phase reaction of nickel tetracarbonyl, sulfur dioxide, and oxygen at 100°C:
Ni(CO)4 + SO2 + O2 → NiSO4 + 4CO
Production
Typically, the salt is obtained as a byproduct of copper refining. It can also be created by dissolving nickel metal or nickel oxides in sulfuric acid.
Nickel carbonate is precipitated when aqueous solutions of nickel sulfate react with sodium carbonate. Nickel carbonate is a precursor to nickel-based catalysts and pigments. Ammonium sulfate precipitates Ni(NH4)2(SO4)2•6H2O when added to concentrated aqueous solutions of nickel sulfate. This blue solid is similar to Mohr’s salt, Fe(NH4)2(SO4)2•6H2O.
Uses
Nickel Sulfate is used to make other Nickel compounds, as a mordant, in dyeing and printing textiles, nickel plating, metal coloring, ceramics, and in the production of driers for use in protective coatings.
In the laboratory, nickel sulfate is used. Nickel sulfate is used to regenerate columns used in polyhistidine-tagging, which is useful in biochemistry and molecular biology. NiSO4•6H2O and related hydrates react with ammonia to form [Ni(NH6)6]SO4 and with ethylenediamine to form [Ni(H2NCH2CH2NH2)3]SO4. Because it does not hydrate, it is occasionally used as a calibrant for magnetic susceptibility measurements.
Natural occurrence
Nickel sulfate occurs as a hexahydrate in the rare mineral retgersite. Nickel hexahydrite (Ni,Mg,Fe)SO4•6H2O is the second hexahydrate. Morenosite is a form of heptahydrate that is relatively unstable in the air. Dwornikite (Ni, Fe)SO4•H2O is a very rare mineral that contains monohydrates.