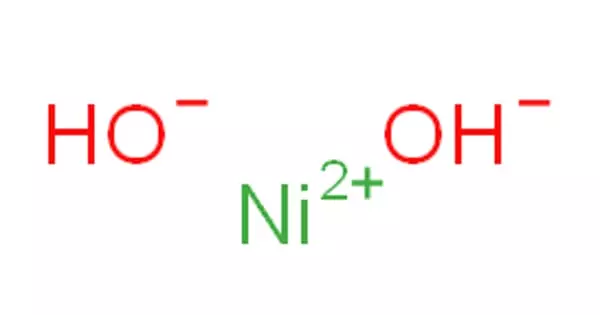
The inorganic compound with the formula Ni(OH)2 is nickel(II) hydroxide. It has the appearance of a fine green powder. It’s an apple-green solid that decomposes in ammonia and amines and is attacked by acids. It is electroactive, and when converted to Ni(III) oxy-hydroxide, it finds widespread use in rechargeable batteries.
It has a slightly higher density than water and is slightly soluble in it. It is an insoluble compound with strong redox properties that has a wide range of industrial and laboratory applications. The primary risk is a threat to the environment. Immediate action should be taken to limit the spread of the disease to the environment.
Properties
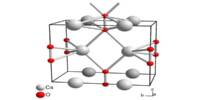
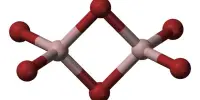
There are two well-known polymorphs of nickel(II) hydroxide, α, and β. The α structure is made up of Ni(OH)2 layers with anions or water intercalated. The β form is composed of Ni2+ and OH ions in a hexagonal close-packed structure. When exposed to water, the polymorph typically recrystallizes to the β form. In addition to the α and β polymorphs, several γ nickel hydroxides with crystal structures with much larger inter-sheet distances have been described.
- Molecular Weight: 92.71
- Appearance: green crystals
- Melting Point: 230° C (446° F)
- Boiling Point: N/A [100ºC at 760 mmHg]
- Density: 4.10 g/cm3
- Solubility in H2O: N/A
- Exact Mass: N/A
- Form: Powder
- Color: Green
The mineral form of Ni(OH)2, theophrastite, was first identified in the Vermion region of northern Greece, in 1980. It is found naturally as a translucent emerald-green crystal formed in thin sheets near the boundaries of idocrase or chlorite crystals. A nickel-magnesium variant of the mineral, (Ni, Mg)(OH)2 had been previously discovered at Hagdale on the island of Unst in Scotland.

Preparation
Nickel hydroxide is made in a variety of ways, the most common of which involve the reaction of caustic soda or caustic potash with a soluble nickel salt. Thus, treating nickel sulfate solution with sodium hydroxide results in the formation of a dense green gel. When the gel is stored for an extended period of time, it crystallizes. Alternatively, after neutralization, the solution forms a fine precipitate of nickel hydroxide. Nickel nitrate is also used as a starting material in the production of nickel hydroxide. Its aqueous solution, when treated with sodium or potassium hydroxide, produces a gelatinous precipitate of nickel hydroxide, which can then be extracted with hot alcohol to yield a high purity product.
An electrolytic process using metallic nickel as the anode and nickel nitrate solution as the electrolyte produces high purity nickel hydroxide. An inert cathode is used to electroplate nickel hydroxide.
Reactions
Nickel(II) hydroxide is commonly used in electric vehicle batteries. In particular, Ni(OH)2 readily oxidizes to nickel oxyhydroxide, NiOOH, when combined with a reduction reaction, frequently involving a metal hydride (reactions 1 and 2).
Reaction 1 Ni(OH)2 + OH– → NiO(OH) + H2O + e–
Reaction 2 M + H2O + e– → MH + OH–
Net Reaction (in H2O) Ni(OH)2 + M → NiOOH + MH
Of the two polymorphs, α-Ni(OH)2 has a higher theoretical capacity and thus is generally considered to be preferable in electrochemical applications. However, it transforms to β-Ni(OH)2 in alkaline solutions, leading to many investigations into the possibility of stabilized α-Ni(OH)2 electrodes for industrial applications.
Applications
By oxidation to nickel(III) oxide-hydroxide, it is most commonly used in rechargeable battery electrodes. Nickel(II) Hydroxide is a common component of electrochemical cells.