Nickel tungstate is an inorganic compound of nickel, tungsten and oxygen, with the chemical formula of NiWO4. It is a member of the tungstate family, a group of compounds that include metal and tungstate (WO₄²⁻) ions. It is relatively insoluble in water, as are many tungstate compounds. It can exhibit notable electrical conductivity under certain conditions, making it of interest in certain technological applications, such as electronic materials and catalysts.
Preparation
Nickel tungstate can be prepared by the reaction of nickel(II) nitrate and sodium tungstate:
Ni(NO3)2 + Na2WO4 → NiWO4 + 2 NaNO3
Nickel tungstate can also be prepared by the reaction of nickel(II) oxide and tungsten(VI) oxide.
It can also be obtained by the reaction of ammonium metatungstate and nickel(II) nitrate or from the reaction of sodium tungstate, nickel(II) chloride and sodium chloride.
Nickel tungstate undergoes a phase transition at 700°C.
Properties
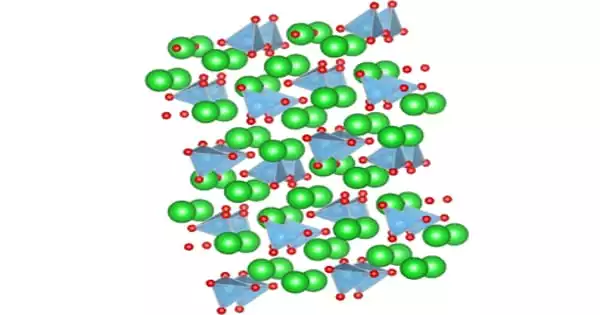
Nickel tungstate is a light brown, odourless solid that is insoluble in water. The amorphous form is green and the polycrystalline form is brown. It crystallizes in the wolframite crystal structure of the monoclinic crystal system with space group P2/c (No. 13). The compound is antiferromagnetic.
- Chemical formula: NiWO4
- Molar mass306.534
- Appearance: green crystals
- Odor: odourless
- Density: 3.3723 g/cm³
- Melting point: 1420 ˚C
- Solubility in water: insoluble
- Solubility: soluble in ammonia
Nickel Tungstate is stable at room temperature but reacts with acids to release tungsten species. It may also undergo reduction to metallic nickel under reducing conditions. The compound is thermally stable up to high temperatures but may decompose under extreme heat.
Applications
Nickel tungstate has no commercial uses. It has been examined as a photocatalyst, in humidity sensors, and in dielectric resonators. It is also considered as a “promising” cathode material for asymmetric supercapacitors.
- Catalysis: It has been studied for its catalytic properties in reactions like hydrogenation and oxidation processes, particularly in petroleum refining.
- Electronics: Due to its electrical conductivity, it can be used in certain electronic applications, especially in the context of thin films and coatings.
- Material Science: Nickel Tungstate has been explored in the development of materials with high-temperature resistance, serving as a component in some specialized alloys.
- Tungsten Recovery: It serves as a precursor in tungsten-based processes or for the recovery of tungsten, often as a byproduct.
Occurrences
Nickel Tungstate is a synthetic compound and is not typically found in nature as a distinct mineral, unlike other tungstates like scheelite (CaWO₄) or ferritungstate (FeWO₄). However, trace amounts may be found in nickel or tungsten ores, where tungsten minerals are associated with nickel.
- Nickel Mining: In nickel-rich ore bodies, such as those found in Canada, Russia, and Indonesia, nickel tungstate may form in small quantities as a secondary mineral.
- Tungsten Mining: When extracting tungsten from ore, nickel tungstate may be present as a byproduct, especially in ores that have nickel impurities.
Safety Considerations
- Toxicity: Nickel compounds are generally considered to be hazardous to health if inhaled or ingested, and long-term exposure may lead to respiratory issues or other health concerns.
- Environmental Impact: Given the toxicity of both nickel and tungsten in some forms, proper disposal and handling practices are recommended when working with nickel tungstate.