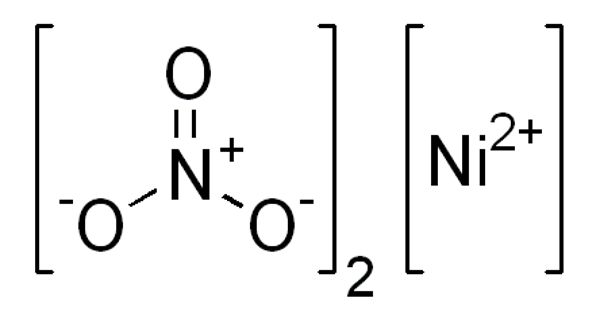Nickel nitrate is a green crystalline solid. It is the inorganic compound Ni(NO3)2 or any hydrate thereof. It is soluble in water. It is noncombustible, but it will accelerate the burning of combustible materials. The anhydrous form is not commonly encountered, thus “nickel nitrate” usually refers to nickel(II) nitrate hexahydrate.
Nickel element (atomic symbol: Ni) has atomic number 28. It is a Block D, Group 4, Period 4 element with an atomic weight of 58.6934. The number of electrons in each of nickel’s shells is 2, 8, 16. Its melting point is 56.7∘C and its boiling point is 136.7∘C. It is soluble in Ethanol.
Preparation
Nickel nitrate hexahydrate may be prepared by several methods based on the reaction of dilute nitric acid on nickel powder, nickel oxide, or nickel carbonate. It is prepared by the reaction of nickel oxide with nitric acid:
NiO + 2 HNO3 + 5 H2O → Ni(NO3)2.6H2O
The anhydrous nickel nitrate is typically not prepared by heating the hydrates. The reaction is exothermic and requires controlled cooling during production. Rather is generated by the reaction of hydrates with dinitrogen pentoxide or of nickel carbonyl with dinitrogen tetroxide:
Ni(CO)4 + 2 N2O4 → Ni(NO3)2 + 2 NO + 4 CO
The hydrated nitrate is often used as a precursor to supported nickel catalysts. The hexahydrate can be dehydrated to anhydrous salt by treatment with fuming nitric acid.

Safety
Like other nitrates, nickel nitrate is oxidizing. It is also irritating to the eyes, skin, and, upon inhalation of the dust, respiratory tract. It may cause skin allergies. If large quantities are involved in a fire or the combustible material is finely divided, an explosion may result.
Nickel nitrate is a carcinogen, along with most other nickel compounds. Prolonged exposure to fire or heat may result in an explosion. Toxic oxides of nitrogen are produced in fires involving this material. The nickel ion is also toxic to aquatic organisms.
Uses
Nickel(II) nitrate is primarily used in electrotyping and electroplating metallic nickel. It is used in a nickel plating and to make nickel catalysts for use in chemical manufacture. It also is used to make nickel plates in nickel-cadmium batteries. Other applications are in ceramics to produce brown colors and in preparing nickel oxide.
Information Source: