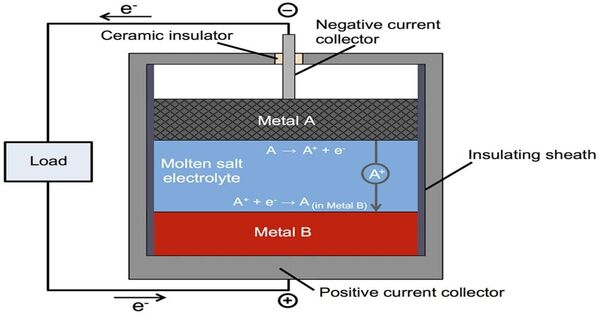
Molten-salt batteries are a type of battery that uses molten salts as an electrolyte and has both a high energy density and power density. It is also known as liquid-metal batteries, which are rechargeable batteries that use a molten salt mixture as the electrolyte. Traditional non-rechargeable thermal batteries can be kept solid at room temperature for extended periods of time before being activated by heating. These batteries typically have three layers: two liquid metal electrodes and a molten salt electrolyte layer.
Rechargeable liquid-metal batteries are utilized as industrial power backup, customized electric vehicles, and grid energy storage to balance out intermittent renewable power sources like solar panels and wind turbines. Molten salts were shown to be effective electrolytes for high-energy rechargeable lithium metal batteries in 2023.
Here’s how they generally work:
- Electrodes: The two electrodes are made of liquid metals, often a molten alloy of different metals. These metals are chosen for their ability to maintain their liquid state at the operating temperature of the battery.
- Electrolyte: The molten salt electrolyte is typically a mixture of different salts. This electrolyte facilitates the movement of ions between the two electrodes during charging and discharging.
- Operation: During charging, ions move from one electrode to the other through the molten salt electrolyte, storing energy. During discharging, the process is reversed, releasing stored energy.
Molten-salt batteries have several potential advantages:
- High Energy Density: Molten-salt batteries can potentially achieve high energy densities, making them suitable for applications requiring large amounts of stored energy, such as grid-scale energy storage.
- Long Cycle Life: They have the potential for long cycle lives, meaning they can be charged and discharged many times before experiencing significant degradation.
- High Efficiency: Molten-salt batteries can have high charge/discharge efficiency, meaning they can effectively convert input energy into stored energy and vice versa.
- Safety: Some molten-salt battery designs are intrinsically safer than other battery chemistries because they operate at high temperatures, preventing thermal runaway reactions.
These batteries have been investigated for a variety of applications, including grid-scale energy storage, where their high energy density and long cycle life may be very beneficial. However, problems persist, including high working temperatures, material compatibility, and cost. Research and development efforts are ongoing to increase the performance and viability of molten-salt battery technology.