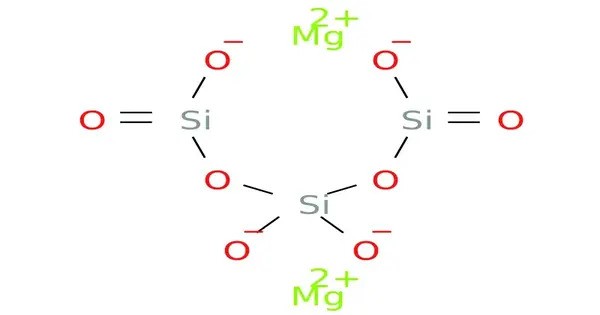
Magnesium trisilicate is an inorganic compound that is used as a food additive. It is a hydrated magnesium salt of silicic acid, appearing as a fine, white, odorless powder. The additive is frequently used by fast food chains to absorb fatty acids and extract impurities formed while frying edible oils. This compound is insoluble in water but swells upon contact with it, forming a gel-like consistency.
It has good acid neutralizing properties, but the reaction appears too slow to serve as an effective non-prescription antacid. It is widely used as an antacid in pharmaceutical formulations. When ingested, magnesium trisilicate reacts slowly with gastric acid (hydrochloric acid) to produce magnesium chloride and silicic acid, neutralizing excess acidity and providing a protective coating on the stomach lining. Its slower reaction compared to other antacids prolongs its effect, making it suitable for relief of heartburn, indigestion, and peptic ulcers.
Properties
- Chemical formula: Mg2O8Si3
- Molar mass: 260.857 g·mol−1
- Appearance: White crystals
- Odor: Odourless
Preparation
It is typically prepared by reacting magnesium salts (such as magnesium sulfate) with sodium silicate under controlled conditions, followed by washing and drying. Its combination of antacid action, low toxicity, and gelling properties makes it a valuable compound in both medical and industrial applications.
Occurrences
Magnesium trisilicate does not occur naturally in pure form but is synthesized industrially from magnesium salts (like magnesium sulfate) and sodium silicate solutions. In nature, related magnesium silicate minerals occur, such as talc, serpentine, and olivine, but these are structurally different from the pharmaceutical-grade material. The compound is widely used in medicine (especially as an antacid for relieving gastric acidity), in food processing (as an additive), and sometimes in industrial applications for filtration or as a filler.
Application
In addition to medicinal uses, magnesium trisilicate can serve as an adsorbent and filler in tablet manufacturing. Its safety profile is generally good, though excessive intake may lead to laxative effects due to magnesium ions or, in rare cases, kidney issues with prolonged high doses.
Health effects
On March 12, 2007, Chinese health authorities halted the use of magnesium trisilicate at Shaanxi Province KFC franchises, suspecting it to be a possible carcinogen. As a response, China’s Ministry of Health conducted tests at six outlets of KFC. The results showed chemicals in the cooking process at KFC restaurants in the country were not harmful. The Ministry of Health said tests showed that using the product to filter cooking oil had no apparent impact on health. Food scares regularly sweep the Chinese media.